More Actionable Targets Improve Therapy in NSCLC
Advances in targeted therapies show encouraging activity as treatment for tough-to-target driver alterations in non–small cell lung cancer emerge, according to data presented at the 2019 ASCO Annual Meeting. The discovery of additional oncogenic drivers and promising targeted therapies means that certain patients will receive treatments that produce favorable outcomes based on their disease characteristics.
Mark A. Socinski, MD
Advances in targeted therapies show encouraging activity as treatment for tough-to-target driver alterations in nonsmall cell lung cancer (NSCLC) emerge, according to data presented at the 2019 American Society of Clinical Oncology (ASCO) Annual Meeting. The discovery of additional oncogenic drivers and promising targeted therapies means that certain patients will receive treatments that produce favorable outcomes based on their disease characteristics.
“There is no greater thing that oncologists can do than to diagnose an oncogenic driver in their patients because it opens up the door to targeted therapy,” Mark A. Socinski, MD, said in an interview with Targeted Therapies in Oncology (TTO). Socinski is executive director of the AdventHealth Cancer Institute in Orlando, Florida.
“It is true that these molecular subgroups represent small slices of the NSCLC cancer pie, but NSCLC is a prevalent cancer. Even when you have a small subset of lung cancer driven by a particular molecular alteration, that translates into a very substantial number of patients,” Ferdinandos Skoulidis, MD, PhD, an assistant professor in the Department of Thoracic/Head and Neck Medical Oncology at The University of Texas MD Anderson Cancer Center in Houston, Texas, said in an interview with TTO (FIGURE 1).1
Targeting the Unmet Need in EGFR-Mutated Tumors
First-, second-, and third-generation EGFR tyrosine kinase inhibitors (TKIs) have been the standard of care for the 15% to 20% of patients who harborEGFRmutations, but additional research has revealed heterogeneity among these mutations. For example, mutations in exons 19 and 21 are common and highly responsive to EGFR TKIs. “A mix of other mutations, some of which are sensitivity mutations and some of which are resistance mutations,” do not respond to any of the currently FDA-approved targeted therapies, Socinski said.
The oral EGFR/HER2 inhibitor TAK-788 exhibits potent and selective preclinical inhibitory activity againstEGFRexon 20 insertions. This relatively uncommon variant accounts for roughly 6% of allEGFRmutations and is generally associated with resistance to available EGFR TKIs. In a multicohort phase I/II trial of patients with advanced NSCLC, 28 patients with an exon 20 insertion who previously received systemic therapy were treated with 160 mg of TAK-788; 43% experienced a confirmed objective response comprising all partial responses (PRs). The confirmed objective response rate (ORR) for patients without central nervous system (CNS) involvement was 56% compared with 25% for patients with baseline metastases. Median progression-free survival (PFS) was 7.3 months across all patients, and patients with and without CNS metastases had a median PFS of 3.7 months and 8.1 months, respectively.2
Toxicity was analyzed from all patients on the trial, and grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 63% of patients treated at the 160-mg dose and 61% of those treated with TAK-788 at any dose. The most common any-grade treatment-related AEs (TRAEs) among patients who received 160 mg daily were diarrhea (85%), nausea (43%), and rash (36%).2
“The challenge in designing drugs forEGFRinsertion mutations resides around sparing the wild-typeEGFR,” Socinski said. “Whereas drugs like osimertinib [Tagrisso] have been designed to have less wild-type EGFR inhibition and therefore less toxicity, particularly skin toxicity, these agents targeting exon 20 insertions have more wild-type EGFR inhibition, so they tend to have more gastrointestinal and skin toxicity than we see with osimertinib.”
Poziotinib blocks signaling throughHER1,2, and4and has shown promise in treating tumors with bothEGFRandHER2exon 20 insertions. An ongoing phase II study is examining the efficacy of poziotinib at reducing tumor size in patients withEGFR- orHER2-positive exon 20 insertion mutations.3 “We will have much more data on poziotinib in the next 6 months or so,” said Socinski, an investigator on the trial. “We have completed a number of different cohorts but have not read out the efficacy yet.”
Poziotinib has shown promising efficacy in patients with metastaticEGFRandHER2exon 20mutant NSCLC, with a best response rate of 56%, according to phase II study results presented at the International Association for the Study of Lung Cancer 19th World Conference on Lung Cancer in Toronto, Canada.4
Another study presented at ASCO that had positive data forEGFRexon 20 insertions involved JNJ-61186372 (JNJ-372), a bispecific antibody that binds and inhibits bothEGFRandcMET.5“Of more relevance to mutant EGFR receptors, [JNJ-372 has] the ability…to degrade the receptor and inhibit downstream signaling,” said Eric B. Haura, MD, who presented the data. This substance has a manageable safety profile and demonstrated preliminary responses in a small group of patients, including those with third-generation TKI-relapsed NSCLC and those with exon 20 insertions.
Patients in the latter group (n = 27) had a PR rate of 30%. Across all patients withEGFRmutations (n = 108) and those with progression on a third-generation TKI (n = 58), 30% and 28% of patients, respectively, had a PR as their best response. The most frequent TEAEs included infusion-related reaction (62%), rash (56%), paronychia (26%), and constipation (22%).5
“JNJ-372 activity is observed across diverseEGFR-mutated lung cancers, including those with unmet medical need,” Haura said. “The safety profile is manageable. The grade 3 and above [treatment-related] toxicity rate is low, at 9%.”
Patients treated with first- and second-generation EGFR inhibitors who develop resistance mutations for which there is no FDA-approved third-generation agent, such as osimertinib (Tagrisso) inEGFRT790M, represent a significant unmet need (FIGURE 2). These patients may now see success with U3-1402, an antibodydrug conjugate targetingHER3with broad activity against resistance mechanisms to EGFR inhibitors.6
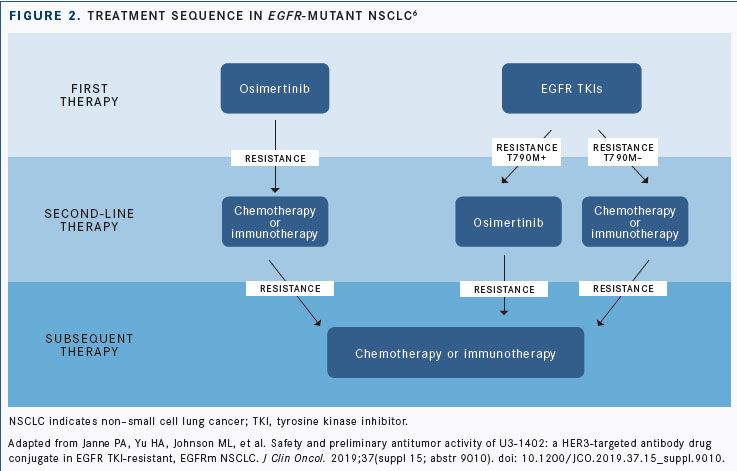
In a phase I trial, patients withEGFR-mutant metastatic or unresectable NSCLC who were T790M negative after receiving erlotinib (Tarceva), gefitinib (Iressa), or afatinib (Gilotrif) or who progressed following osimertinib were eligible for inclusion in the dose-escalation/expansion study. All patients evaluable by immunohistochemistry were positive forHER3expression, and the most commonEGFR-activating mutation was exon 19 deletion (13 of 23, 56.5%), followed by L858R (39.1%) and L861Q (4.3%).6
Antitumor activity across diverse EGFR TKI resistance mechanisms in 16 evaluable patients showed 4 partial responses, 2 of which occurred in patients with the known osimertinib resistance mechanism C797S. Most TEAEs were grade 1/2.6
“Based on these findings, we believe that targeting HER3 with U3-1402 may be a practical approach to treatEGFR-mutant NSCLC that has developed diverse mechanisms of resistance to EGFR TKIs,” Pasi A. Jänne, MD, PhD, director of the Lowe Center for Thoracic Oncology at Dana-Farber Cancer Institute in Boston, Massachusetts, and lead author on the study, told the audience during his presentation at ASCO.
RETRearrangements
Patients may soon be routinely tested forRETrearrangements, according to D. Ross Camidge, MD, PhD, the Joyce Zeff Chair in Lung Cancer Research at the University of Colorado Denver. Camidge’s focus is in thoracic malignancies and developmental therapeutics (phase I studies), and he was an investigator on several therapeutics for use in NSCLC presented at ASCO 2019. Approximately 1% to 2% of NSCLC cases are attributable toRETfusions, and alterations in this gene are also observed at elevated rates in medullary and papillary thyroid cancers. To date, patients with NSCLC andRETfusions have seen little clinical benefit with chemotherapy, immunotherapy, or TKIs.7
No FDA-approved selective RET inhibitors are currently available, but the substance BLU-667, which had data presented at ASCO, showed promise for this indication. Data from the phase I ARROW trial of BLU-667 in patients withRETfusionpositive solid tumors showed theRET-inhibiting agent to be well tolerated. Among patients with advanced NSCLC, the overall response rate was 58%, including 1 complete response (CR) and 27 PRs. Seven of 9 patients (78%) had shrinkage of measurable brain metastases, and none on the starting dose of 400 mg daily experienced progression due to new CNS involvement. Because of treatment-related toxicity, 7% of patients discontinued BLU-667. The RET inhibitor maintained activity regardless of prior checkpoint inhibitor therapy and across RET-fusion genotypes. The most common all-grade TEAEs were constipation (30%), neutropenia (26%), aspartate aminotransferase increase (24%), fatigue (21%), and hypertension (20%).7
BLU-667 now has FDA breakthrough therapy designation forRETfusionpositive NSCLC that has progressed following platinum-based chemotherapy.8
METExon 14 Skipping Mutations
For the 3% to 4% of NSCLC cases that are likely due toMETexon 14 skipping (METex14) mutations, treatment with the highly selective MET TKI tepotinib may become an option, based on positive findings from the phase II VISION trial.9
“We do know that crizotinib [Xalkori] has some activity here and have been using it outside of its FDA label in patients who have this finding, but after some initial enthusiasm, we are finding the activity of crizotinib may not be as great as was formerly thought,” Socinski said.
Tepotinib demonstrated an ORR of 50% in patients with any histology, including squamous and sarcomatoid disease, and a METex14-positive tumor by liquid biopsy. The median duration of response (DOR) was 12.4 months. ForMETex14-positive tumors identified by tissue biopsy, the ORR was 45.1% and the median DOR was 15.7 months. Of the 87 patients treated, 37.9% were treatment naïve, 9.2% had brain metastases, and all had an ECOG performance of ≤1. There were no grade ≥4 TRAEs, and 19.5% of patients experienced ≥1 grade 3 TRAE. The most common all-grade TRAEs were peripheral edema (48.3%), nausea (23.0%), and diarrhea (20.7%).9
In the phase II GEOMETRY mono-1 study of patients withMETex14-positive advanced NSCLC, the MET inhibitor capmatinib (INC280) showed an ORR of 67.9% in treatment-naïve patients. Among pretreated patients (n = 69), including 88.4% who had been treated with platinum-based chemotherapy, the ORR was 40.6% and median PFS was 5.42 months (95% CI, 4.17- 6.97). In the treatment-naïve cohort, the median PFS was 9.69 months (95% CI, 5.52-13.86). With a median treatment exposure time of 14.9 weeks, 84.4% of patients experienced any-grade TRAEs and 35.6% had a grade 3/4 AE.
The most common grade 3/4 events were peri-pheral edema (7.5%) and fatigue (3.0%).10
The most interesting thing about these drugs is that the response to them suggests the possibility of additional heterogeneity, Camidge said. “Almost everybody who is developing a MET inhibitor has [observed close to] a 40% response rate. That proves that MET mutations are driver oncogenes but that responses are lower than what we see with EGFR, ALK, and ROS1, where we get 60% to 70% response rates,” he said. “Is this telling us that what we think is a uniform population is not? [There may be] a group in here that actually has an 80% response rate and a group who does not, and we are lumping them all together.”
Targeting the “Undruggable”KRAS
To date, there are no FDA-approved therapies forKRASmutations. “For a long time, it had been considered undruggable,” Skoulidis said. However, data presented at ASCO suggest that it may be possible to effectively targetKRASG12C, which occurs in about 13% of patients with lung cancer.
According to findings from a multicenter, phase I, first-in-human study, the novel small-moleculeKRASG12C inhibitor AMG 510 was safe and well tolerated in 35 patients with locally advanced or metastaticKRASG12Cmutated NSCLC and other solid tumors without brain metastases. Of 10 patients with NSCLC, 5 achieved a PR as their best response. The agent was well tolerated, and there were no dose-limiting toxicities.11
“That was like an earth tremor,” Camidge said. “Suddenly there is a glimmer of hope10 patients’ worth—that these KRAS mutations may be targetable in the future. Now the question is, if they add another 10 patients in, will the response rate remain at 50% or is it going to drop down?” For now, he says, it is important to determine whether there is relevant molecular heterogeneity in KRAS G12C.
According to Skoulidis, the phase II AMG 510 study was scheduled to begin enrolling patients in the summer of 2019.11
Another ongoing investigation involves the MEK inhibitor trametinib (Mekinist) plus docetaxel in patients withKRAS-mutant NSCLC to determine whether targeting theMEK/ERKpathway may have efficacy in patients with different types ofKRASmutations. The trial met its primary endpoint with a 33% response rate, consisting of all PRs, in the total cohort. The median PFS was 3.3 months in patients withKRASG12Cmutant tumors versus 4.1 months in patients with otherKRASmutations (HR, 1.82; 95% CI, 1.0-3.28), and the median overall survival was 8.8 months and 12.2 months, respectively (HR, 1.57; 95% CI, 0.79-3.13).12
“These data suggest that therapeutic outcomes may vary in different genetic subsets ofKRAS-positive NSCLC,” the investigators wrote in their conclusion.12
AXL Plus PD-L1 Inhibition
In a phase II clinical trial of the AXL inhibitor bemcentinib (BGB324) plus the PD-1 inhibitor pembrolizumab (Keytruda) in patients with immunotherapy-naïve, second-line advanced NSCLC and at least 1 radiologic assessment (n = 35), the combination produced an ORR of 29% in all patients, which was made up of 10 PRs. Patients on the trial were not required to have AXL- or PD-L1positive status to be eligible for inclusion.13
AXL is a member of the TAM family of receptor tyrosine kinases expressed on tumor and immune cells and is overexpressed in response to a hostile tumor microenvironment. Bemcentinib has been shown to improve the efficacy of checkpoint blockade in preclinical models of NSCLC.13
More than half (58%) of patients on the trial were AXL positive. The best responses were observed in this group (ORR, 40%), including in patients with low or no PD-L1 expression.13
Basket Trial Data inCDKN2ALoss/Mutations
The TAPUR study is evaluating the antitumor activity of commercially available targeted agents in patients with specific genomic aberrations and advanced cancers. In this phase II basket study, 28 patients withCDKN2A-altered NSCLC without a standard treatment option received palbociclib (Ibrance). The trial’s design indicated that if at least 7 of the 28 treated patients had stable disease or better, the drug would be worthy of further study for that disease indication.14
Palbociclib resulted in a disease control rate of 29% (90% CI, 15%-37%) and a median PFS of 7.9 weeks (95% CI, 7.0-15.1), leading trial investigators to conclude that “additional study is warranted to confirm the efficacy of [the agent] in patients with NSCLC withCDKN2Aloss or mutations.”
Next-Generation Sequencing: A Clinical Necessity
“If there is a major unifying message that is emerging as a result of this ASCO meeting, it is that broad genomic profiling should be considered the standard of care for the up-front management of patients with advanced or metastatic NSCLC, predominantly adenocarcinoma,” Skoulidis said. “This is already standard practice across almost all academic centers, but it is important to cement this message in the minds of community oncologists, as well.”
In addition to the 5 distinct, molecularly specific subtypes of NSCLCEGFRandBRAFV600E mutations,ALKandROS1rearrangements, andNTRKgene fusionsthat have FDA-approved targeted therapies, the most recent advancements in agents for novel targets may expand the list of aberrations in patients to be tested in the future.
Fortunately, the discovery of additional molecular targets makes it economically advantageous to choose next-generation sequencing (NGS) over individual testing for specific mutations. A 2019 study published in JCO Precision Oncology concluded that up-front NGS testing in patients with metastatic NSCLC was associated with substantial cost savings and shorter time-to-test results.15 “Once you get past 4 analytes, it costs the same to do an NGS panel, which could look at 50 genes, as it does to bill for each individual analyte,” Camidge said.
Waiting for the results of both NGS and PD-L1 testing before instituting a regimen is necessary when there are extensive treatment options for patients with stage IV disease. “You will typically know the PD-L1 status a week or 2 before you know the molecular status, but PD-L1 means nothing until you know the genotype of the tumor,” Camidge said. “A never-smoker who has a PD-L1 expression of 90% has a 40% to 50% chance of having anEGFRmutation. Starting them on immunotherapy is the wrong thing to do if they turn out to have anEGFRmutation.”
Camidge recommended NGS of both tissue and blood. “If you have anEGFRmutation, 15% to 20% of the time you will find it only in the tissue, not in the blood, or only in the blood and not in the tissue,” he said. “It is our job to give patients the most effective therapy for their cancer, and in 2019, that entails knowing the details of their cancer genetics.”
References:
- Shepherd FA. Targeted therapy: the new frontier. Presented at: 2019 American Society of Clinical Oncology Annual Meeting; June 1-4, 2019; Chicago, IL. https://bit.ly/2G1HqmR.
- Janne PA, Neal JW, Camidge DR, et al. Antitumor activity of TAK-788 in NSCLC with EGFR exon 20 insertions. J Clin Oncol. 2019;37(suppl 15; abstr 9007). 10.1200/JCO.2019.37.15_suppl.9007.
- Yang Z, Tchekmedyian N, Chu DT, et al. A phase 2 study of poziotinib in patients with EGFR or HER2 exon 20 mutation-positive non-small cell lung cancer. J Clin Oncol. 2018;36(suppl; abstr TPS9160). bit.ly/2KtgeNQ.
- Heymach J, Negrao M, Robichaux J, et al. A phase II trial of poziotinib in EGFR and HER2 exon 20 mutant non-small cell lung cancer (NSCLC). Presented at: International Association for the Study of Lung Cancer 19th World Conference on Lung Cancer; September 23-26, 2018; Toronto, Ontario, Canada. Abstract OA02.06. bit.ly/2IQDHKN
- Haura EB, Cho BC, Lee JS, et al. JNJ-61186372 (JNJ-372), an EGFR-cMET bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2019;37(suppl 15; abstr 9009). doi: 10.1200/ JCO.2019.37.15_suppl.9009.
- Janne PA, Yu HA, Johnson ML, et al. Safety and preliminary antitumor activity of U3-1402: a HER3-targeted antibody drug conjugate in EGFR TKI-resistant, EGFRm NSCLC. J Clin Oncol. 2019;37(suppl 15; abstr 9010). doi: 10.1200/JCO.2019.37.15_suppl.9010.
- Gainor JF, Lee DH, Curigliano G, et al. Clinical activity and tolerability of BLU-667, a highly potent and selective RET inhibitor, in patients (pts) with advanced RET-fusion+ non-small cell lung cancer (NSCLC). J Clin Oncol. 2019;37(suppl 15; abstr 9008). doi: 10.1200/JCO.2019.37.15_suppl.9008.
- Blueprint Medicines reports first quarter 2019 financial results [news release]. Cambridge, MA: Blueprint Medicines Corporation; May 9, 2019. bit. ly/2xhXOLw. Accessed June 27, 2019.
- Paik PK, Veillon R, Cortot AB, et al. Phase II study of tepotinib in NSCLC patients with METex14 mutations. J Clin Oncol. 2019;37(suppl 15; abstr 9005). 10.1200/JCO.2019.37.15_suppl.9005.
- Wolf J, Seto T, Han J-Y, et al. Capmatinib (INC280) in METΔex14-mutated advanced non-small cell lung cancer (NSCLC): 3fficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol. 2019;37(suppl 15; abstr 9004). doi: 10.1200/JCO.2019.37.15_suppl.9004.
- Fakih M, O’Neil B, Price TJ, et al. Phase 1 study evaluating the safety, tolerability, pharmacokinetics (PK), and efficacy of AMG 510, a novel small molecule KRASG12C inhibitor, in advanced solid tumors. J Clin Oncol. 2019;37(suppl 15; abstr 3003). doi: 10.1200/JCO.2019.37.15_suppl.3003.
- Gadgeel SM, Miao J, Riess JW, et al. S1507: phase II study of docetaxel and trametinib in patients with G12C or non-G12C KRAS mutation positive (+) recurrent non-small cell lung cancer (NSCLC). J Clin Oncol. 2019;37 (suppl 15; abstr 9021). doi: 10.1200/JCO.2019.37.15_suppl.9021.
- Felip E, Brunsvig P, Vinolas N, et al. A phase II study of bemcentinib (BGB324), a first-in-class highly selective AXL inhibitor, with pembrolizumab in pts with advanced NSCLC: OS for stage I and preliminary stage II efficacy. J Clin Oncol. 2019;37(suppl 15; abstr 9098). doi: 10.1200/ JCO.2019.37.15_suppl.9098.
- Ahn ER, Mangat PK, Garrett-Mayer E, et al. Palbociclib (P) in patients (pts) with non-small cell lung cancer (NSCLC) with CDKN2A alterations: results from the Targeted Agent and Profile Utilization Registry (TAPUR) study. J Clin Oncol. 2019;37(suppl 15; abstr 9041). doi: 10.1200/ JCO.2019.37.15_suppl.9041.
- Pennell NA, Mutebi A, Zhou Z-Y, et al. Economic impact of next-generation sequencing versus single-gene testing to detect genomic alterations in metastatic nonsmall-cell lung cancer using a decision analytic model [published online May 16, 2019]. JCO Precis Oncol. doi: 10.1200/PO.18.00356.