Unique Immunotherapy Combinations Provide an Optimistic Outlook in Early Urothelial Carcinoma Trials
Ongoing research seeks to boost objective response rates in urothelial cancer by developing biomarkers to predict which immune checkpoint inhibitors a patient is most likely to respond to and by testing these in combination with each other and other treatment types to increase both the number and duration of responses.
Robert Li, MD
Immune checkpoint inhibitors (ICIs) have demonstrated significant and lasting benefits in some patients with urothelial cancer (UC), but objective response rates to ICI monotherapy have only been 14.5% to 24%, and only about half of those patients experience long-term remissions.1,2
Ongoing research seeks to boost those numbers by developing biomarkers to predict which ICI a patient is most likely to respond to and by testing ICIs in combination with each other and other treatment types to increase both the number and duration of responses.
The combinations being tested pair medicines that bind to programmed death receptor 1 (PD-1) or its ligand (PD-L1) with a wide variety of immunotherapies: cytotoxic T-lymphocyteassociated antigen-4 (CTLA-4) inhibitors, indoleamine 2,3-dioxygenase 1 (IDO1) inhibitors, 4-1BB inhibitors, OX40 agonists, antibody–drug conjugates (ADCs), T-cell immunoreceptor with immunoglobulin and ITIM domains (TIGIT) inhibitors, and lymphocyte activation gene-3 (LAG3) inhibitors. Other research is testing PD-1/ L1 blockades with chemotherapies; radiation and targeted therapies, such as fibroblast growth factor receptor (FGFR) inhibitors cabozantinib (Cabometyx) and acalabrutinib (Calquence); and other medications suggested by patient biomarkers.
“The approval of checkpoint inhibitor monotherapy has already had a very significant impact on the treatment of urothelial cancer, which had not seen any entirely new treatments for several decades before they came along. Bladder cancers benefitted minimally from the advent of targeted therapies because they are so heterogeneous on the molecular level that they do not really share any druggable targets. The finding that PD-1/L1 inhibitors produced durable responses in a significant minority of patients was a major breakthrough,” said Roger Li, MD, a genitourinary oncologist at Moffitt Cancer Center in Tampa, Florida.
“Now there is evidence that we can boost response rates and durations even further by pairing PD-1/L1 inhibitors with other medications [that] enhance their effect by potentiating the immune response,” said Li, who is participating in several trials of UC or bladder cancer treatments. “The idea is to create an immunogenic microenvironment so that high numbers of immune cells are around when you add the checkpoint inhibitor to stimulate even more aggressive attacks on the cancer.”
UC, which originates in the bladder or upper urinary tract, is the most common histological type of cancer. It constitutes the majority of all types of bladder cancer, which is diagnosed in about 81,000 Americans each year and accounts for more than 17,000 annual deaths.1Cisplatin-based combination chemotherapy has long been the standard of care in unresectable and metastatic/advanced UC. Such treatment produces an overall response rate (ORR) of 40% to 50% and a median overall survival (OS) of 14 to 15 months.2,3However, issues such as impaired renal function or poor performance status prevent up to half of patients with metastatic UC from qualifying for cisplatin-based chemotherapy. Such patients typically receive carboplatin-based regimens, which produce an ORR of 30% to 40% and OS of 9 to 10 months.3,4 Second-line chemotherapies, including paclitaxel, pemetrexed (Alimta), docetaxel, and vinflunine, have shown a median OS of 5 to 7 months.3,5,6
Recent years have seen the FDA approve 5 ICIs for UC treatment. Pembrolizumab (Keytruda) and nivolumab (Opdivo) both block PD-1, and atezolizumab (Tecentriq), durvalumab (Imfinzi), and avelumab (Bavencio) all block PD-L1. All 5 have undergone trials as second-line treatment in platinum-refractory patients with UC. ORRs have ranged from 14.8% for atezolizumab7 to 21.1% for pembrolizumab,8and the median OS has ranged from 6.5 months for avelumab9to 18.2 months for durvalumab.10Atezolizumab and pembrolizumab have also undergone trials as first-line treatment in platinum-ineligible patients. Atezolizumab produced an ORR of 23% and median OS of 15.9 months,11and pembrolizumab produced an ORR of 24%; OS results have not matured yet.12
Benefits of Combination Therapy
Research in other tumor types has shown that PD-1/L1 blockades produce more numerous and more durable responses when used in combination with other medications than when used as monotherapies. The most successful of those multidrug regimens combine PD-1/L1 blockades with CTLA-4 inhibitors, and early trial data suggest that such combinations may also benefit patients with UC.
Still more early stage trials are assessing the safety and preliminary activity of regimens that combine ICIs with personalized vaccines that take tumor samples from individual patients, find antigens that occur on the tumors but not in a patient’s healthy cells, and teach the immune system to attack those antigens.
Preliminary results from the phase I/II CheckMate 032 trial found that combination treatment with nivolumab and ipilimumab (Yervoy) was associated with an ORR of 26% to 38.5% and a complete response (CR) rate of 2.9% to 3.8% in 208 patients with locally advanced or metastatic UC that had previously been treated with a platinum-based combination. Most responses occurred early, and 70% to 80% of patients showed ongoing response at the time of analysis. Most treatment-related adverse events (AEs) were low grade and manageable.13
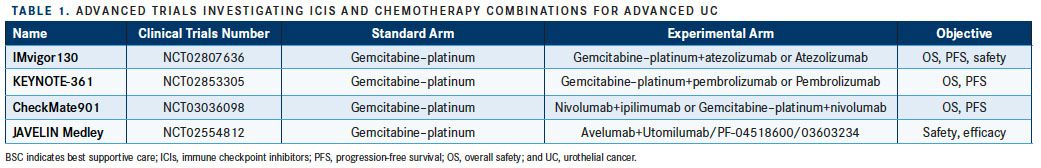
The phase III CheckMate 901 study (NCT03036098) will compare standard first-line platinum-based chemotherapy, alone or with nivolumab, versus nivolumab 1 mg/kg plus ipilimumab 3 mg/kg for 4 cycles followed by nivolumab monotherapy in patients with treatment-naïve metastatic or unresectable UC (TABLE 1). Another randomized phase III trial, DANUBE (NCT02516241), will pit both durvalumab monotherapy and a combination of durvalumab and tremelimumab against standard first-line platinum chemotherapy in patients with metastatic or unresectable UC.
IDO1 inhibitors
Other possible complements for PD-1/L1 inhibitors in patients with UC are medications that bind to IDO1, an intracellular enzyme that creates an immunosuppressive microenvironment by inducing tryptophan degradation and kynurenine production. Earlier study results suggest some solid tumors may upregulate IDO1 to evade immune response, and some investigators have hypothesized that targeting both the PD-1/L1 and IDO1 pathways would enhance outcomes.
KEYNOTE-037 is an ongoing phase I/II study evaluating the safety and efficacy of the oral IDO1 inhibitor epacadostat in combination with pembrolizumab across multiple tumors (NCT02178722). Preliminary results from 40 patients in the UC expansion cohort showed that combination therapy was associated with an ORR of 35%. Patients who were PD-L1positive (those with a combined positive score ≥1%) experienced an ORR of 64%, while patients who were PD-L1– negative experienced a 13% ORR. Median duration of response (DOR) was 30.6 weeks. Grade 3/4 AEs occurred in 22.5% of patients, and the overall safety profile of the combination was comparable to that of pembrolizumab monotherapy.14,15
The combination is currently being studied in 2 phase III trials: the KEYNOTE-672 study, which is comparing pembrolizumab with either epacadostat or placebo in 650 untreated, cisplatin-ineligible patients with advanced UC (NCT03361865), and the KEYNOTE-698 study, which will test the same treatments in 648 patients with advanced UC who have already failed on first-line platinum chemotherapy (NCT03374488). There is, moreover, at least 1 other study designed to test a PD-1/L1 inhibitor (nivolumab) combined with an IDO1 inhibitor (BMS-986205). The phase I trial will test safety and ORR in 18 patients with UC (NCT03192943).
Another drug that might enhance the efficacy of PD-1/L1 inhibitors in the treatment of patients with UC is utomilumab, a monoclonal antibody that increases T-cell proliferation and cytokine production by binding to the 4-1BB receptor on natural killer cells, CD8-positive T cells, and CD4-positive T cells. The KEYNOTE-0036 phase Ib trial of utomilumab and pembrolizumab in 23 patients with various advanced solid tumors reported no dose-limiting toxicities or treatment related discontinuations and an ORR of 26.1%.16 Those results, combined with strong activity in preclinical trials, have led investigators to design phase II trials of utomilumab and pembrolizumab in patients with UC. Similar trials could also test combinations of utomilumab and avelumab, pending positive results from the JAVELIN Medley study (NCT02554812) and the NCT03217747 study in patients with many solid tumor types.
Other early-stage trials have produced some response in patients with UC who take combinations of utomilumab and the OX40 inhibitor PF-04518600. OX40 is a costimulatory immune checkpoint receptor that is expressed on activated CD4-positive T cells and regulatory T cells (Tregs). Preclinical trial results have shown that agonistic monoclonal antibodies that bind to OX40 can reduce the inhibitory effect of Tregs in the tumor microenvironment, and that work has led to early stage clinical trials such as NCT02315066, which is testing utomilumab and PF-04518600 in 42 patients, including 5 with previously treated UC. Among the 37 patients who were evaluable when investigators calculated preliminary results, the ORR was just 5.4%; however, the stable disease rate was 29.7%. Among the 4 evaluable patients with UC, 2 experienced stable disease and 2 experienced progressive disease. Treatment-related AEs were mostly grade 1/2.17 These preliminary results have convinced investigators to continue the trial to evaluate the combination in patients who do not respond (or have stopped responding) to other ICIs. The JAVELIN Medley study and NCT03217747, moreover, are evaluating the safety and preliminary activity of avelumab plus PF-04518600, with or without utomilumab or radiation, in patients with UC and other solid tumors.
AntibodyDrug Conjugates
A different class of medications that might team effectively with ICIs in UC treatment are ADCs, which combine antibodies designed to bind with tumor-surface antigens and toxic drugs that are released after binding has taken place.
A phase I/II study has tested the safety and activity of the ADC sacituzumab govitecan in patients with previously treated advanced solid tumors, including an expansion cohort of 41 patients with advanced UC (NCT01631552).18,19Patients with a median of 3 prior therapies (range, 1-6), including platinum combinations (93%) and ICIs (34%), received 10 mg/kg on days 1 and 8 of 21-day cycles until progression or unacceptable toxicity.15Treatment was associated with an ORR of 34%, and CT scans showed at least some shrinkage of the target lesion in 72% of patients. The median DOR was 12.9 months (95% CI, 7.5-12.9), median PFS was 7.2 months (95% CI, 5.0-10.7), and median OS was 16.1 months (95% CI, 10.5-17.7). The most common grade 3/4 AEs were neutropenia (39%), anemia (10%), fatigue (7%) and diarrhea (7%).15,19
A phase I dose-escalation study evaluated the safety and efficacy of a different ADC, enfortumab vedotin, in 81 patients with advanced UC.20 More than 60% had received ≥2 prior therapies, 45% had prior platinum combinations, and 46% had undergone prior ICI therapy.15The ORR was 41%, and the disease control rate (partial response plus CR plus stable disease) was 72%. Median DOR was 24 weeks (range, 0.14-40.3). Those results spurred a dose-expansion cohort in 67 patients with advanced UC who had already been treated with ICIs. The ORR in that group was 54%.21These findings led to a phase Ib dose-escalation study of enfortumab vedotin in combination with pembrolizumab or atezolizumab in patients with advanced UC pretreated with platinum-based chemotherapy or who were cisplatin ineligible (NCT03219333).
TIGIT and LAG3
Other early-stage trials are investigating combinations of PD-1/L1 inhibitors and drugs that block the TIGIT pathway. BMS-986207, a monoclonal antibody targeting TIGIT, is currently being investigated in a phase I/IIa trial in combination with nivolumab in advanced solid tumors, including UC (NCT02913313). Another monoclonal antibody that binds to TIGIT, MTIG7192A, is being evaluated as a monotherapy and in combination with atezolizumab in a 2-step phase I study in patients with UC and a variety of other advanced tumors (NCT02794571).
Trials that combine PD-1/L1 inhibitors with ICIs that inhibit LAG3 are also at the phase I/II stage in patients with UC and other tumor types. LAG3 is expressed on tumor-infiltrating T lymphocytes, and it is thought that blocking the LAG3 receptor may enhance immune response to tumors in a way that complements a blockade of PD-1, which is also expressed on tumor-infiltrating T lymphocytes.
Targeted Therapies
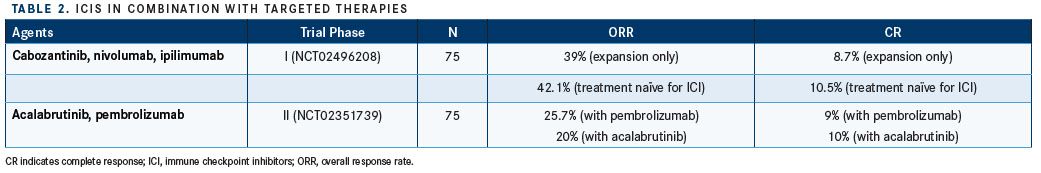
Another major strategy for increasing both the number of responses to PD-1/L1 inhibitors and the duration of those responses is pairing the ICIs with targeted therapies such as cabozantinib and acalabrutinib, FGFR inhibitors, and other medications selected via tumor biomarkers (TABLE 2).
Cabozantinib is a tyrosine kinase inhibitor that targets VEGFR2, MET, RET, FLT3, cKIT, and AXL. A phase I expansion study in 75 patients with metastatic UC and other genitourinary cancers initially split patients among 7 regimens before giving them cabozantinib 40 mg daily, nivolumab 1 mg/kg, and ipilimumab 3 mg/kg in the expansion part of the study.15 Among the patients who had not previously received an ICI, the ORR was 42.1%, the CR rate was 10.5%, and the clinical benefit rate was 84.2%. The UC cohort had a median PFS of 12.8 months (95% CI, 1.8-undefined) and a 12-month OS rate of 70.2% (95% CI, 44.4%-85.8%).22
Acalabrutinib is a second-generation inhibitor of the Bruton tyrosine kinase. The phase II KEYNOTE-143 trial (NCT02351739) is investigating its effect, when paired with pembrolizumab, in platinum-refractory UC. Preliminary results for 75 patients randomized between pembrolizumab alone and a pembrolizumabacalabrutinib combination report a higher ORR for the monotherapy (25.7% vs 20%), but among responders, the combination was more likely to produce a response that lasted at least 12 months (63.5% vs 83.3%).23
Other agents that inhibit FGFRs, which is amplified in UC and other solid tumors, are also being tested in combination with ICIs. The BISCAY phase Ib trial includes a module that combines durvalumab with AZD4547, an inhibitor of FGFR-1, -2, and -3, in patients presenting FGFR3 mutations or FGFR fusions (NCT02546661). Another 2 clinical trials are assessing the safety and efficacy of pembrolizumab in combination with an anti-FGFR3 monoclonal antibody called B-701 in patients with metastatic UC (NCT02925533, NCT03123055).
The BISCAY trial is also looking at other biomarker-based treatment combinations in patients with metastatic UC. Patients with tumors showing mutations in a homologous recombination repair gene panel will receive durvalumab in combination with the PARP inhibitor olaparib (Lynparza). Patients with tumors showing mutations in genes involved in cell-cycle regulation will receive durvalumab in combination with the WEE1 inhibitor AZD1775. Patients with tumors showing genomic alterations that have potential to respond to mTOR inhibitors (RICTOR amplification, TSC1/2 mutations) will receive durvalumab in combination with vistusertib, a selective mTORC1 and mTORC2 inhibitor. Patients with no specific genomic alterations will receive either durvalumab monotherapy or durvalumab plus the STAT3 inhibitor AZD9150.
And that is not all. Other trials are exploring the effects of combining ICIs with chemotherapy or with radiation.
“Barring some truly incredible results from early-stage trials, we are probably a couple of years away from any results that could change the standards of care,” Li said. “But there is considerable optimism that treatment will improve, because several different combinations have produced promising early-stage results. It also makes theoretical sense that immunotherapies would be effective in urothelial cancers because they have some of the highest metastatic burdens of all tumor types and previous research has shown a correlation between tumor burden and immunotherapy efficacy.”
References:
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. SEER website. seer.cancer.gov/statfacts/html/urinb.html. Accessed February 26, 2019.
- Clark PE, Spiess PE, Agarwal N, et al. NCCN guidelines insights bladder cancer, version 2.2016 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2016;14(10): 1213-1224.
- Alfred Witjes J, Lebret T, Compérat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder dancer. Eur Urol. 2017;71(3):462-475. doi: 10.1016/j.eururo.2016.06.020.
- DeSantis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2): 191-199. doi: 10.1200/JCO.2011.37.3571.
- Galsky MD, Pal SK, Lin SW, et al. Real-world effectiveness of chemotherapy in elderly patients with metastatic bladder cancer in the United States. Bladder Cancer. 2018;4(2): 227-238. doi: 10.3233/BLC-170149.
- Niegisch G, Gerullis H, Lin SW, et al. A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J Cancer. 2018;9(8): 1337-1348. doi: 10.7150/jca.23162.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single arm, phase 2 trial. Lancet. 2016;387(10031): 1909-1920. doi: 10.1016/S0140-6736(16)00561-4.
- Bellmunt J, deWit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11): 1015-1026. doi: 10.1056/NEJMoa1613683.
- Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018;19(1):51-64. doi: 10.1016/S1470-2045(17)30900-2.
- Powles T, O'Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. Updated results from a phase 1/2 open-label study. JAMA Oncol. 2017;3(9): e172411. doi: 10.1001/jamaoncol.2017.2411.
- Balar AV, Galsky MD, Rosenberg JE, et al; IMvigor210 Study Group. Atezolizumab as first-line therapy in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064): 67-76. doi: 10.1016/S0140-6736(16)32455-2.
- Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483-1492. doi: 10.1016/S1470-2045(17)30616-2.
- Rosenberg JE, Sharma P, de Braud F, et al. Nivolumab (N) alone or in combination with ipilimumab (i) in patients (pts) with platinum-pretreated metastatic urothelial carcinoma (mUC), including the nivolumab 1 mg/kg + ipilimumab 3 mg/kg expansion from CheckMate-032. In: Proceedings from the 2018 ESMO Congress; October 19-23, 2018; Munich, Germany. Abstract LBA32. oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/Nivolumab-N-Alone-or-in-Combination-With-Ipilimumab-I-in-Patients-pts-With-Platinum-Pretreated-Metastatic-Urothelial-Carcinoma-mUC-Including-the-Nivolumab-1-mg-kg-Ipilimumab-3-mg-kg-Expansion-From-CheckMate-032.
- Smith DC, Gajewski T, Hamid O, et al. Epacadostat plus pembrolizumab in patients with advanced urothelial carcinoma: Preliminary phase I/II results of ECHO-202/KEYNOTE-037. J Clin Oncol. 2017;35:15(suppl; abstr 4503). http://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.4503
- Rodriguez-Vida A, Perez-Gracia JL, Bellmunt J. Immunotherapy combinations and sequences in urothelial cancer: facts and hopes. Clin Cancer Res. 2018;24(24); 61156124.
- Tolcher AW, Sznol M, Hu-Lieskovan S, et al. Phase Ib study of utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res. 2017;23(18):5349-5357. doi: 10.1158/1078-0432.CCR-17-1243.
- Hamid O, Ros W, Thompson JA, et al. Safety, pharmacokinetics (PK) and pharmacodynamics (PD) data from a phase I dose-escalation study of OX40 agonistic monoclonal antibody (mAb) PF-04518600 (PF-8600) in combination with utomilumab, a 4-1BB agonistic mAb. Presented at: ESMO 2017 Congress; September 8-12, 2017; Madrid, Spain. Abstract 1142PD. oncologypro.esmo.org/Meeting-Resources/ESMO-2017-Congress/Safety-pharmacokinetics-PK-and-pharmacodynamics-PD-data-from-a-Phase-I-dose-escalation-study-of-OX40-agonistic-monoclonal-antibody-mAb-PF-04518600-PF-8600-in-combination-with-utomilumab-a-4-1BB-agonistic-mAb.
- Faltas B, Goldenberg DM, Ocean AJ, et al. Sacituzumab govitecan, a novel antibody-drug conjugate, in patients with metastatic platinum-resistant urothelial carcinoma. Clin Genitourin Cancer. 2016;14(1):e75-e79. doi: 10.1016/j.clgc.2015.10.002.
- Tagawa S, Faltas B, Lam E, et al. Sacituzumab govitecan (IMMU-132) for patients with pretreated metastatic urothelial uancer (UC): interim results. Presented at: ESMO 2017 Congress; September 8-12, 2017; Madrid, Spain. Abstract 858P. oncologypro.esmo.org/Meeting-Resources/ESMO-2017-Congress/Sacituzumab-govitecan-IMMU-132-for-patients-with-pretreated-metastatic-urothelial-uancer-UC-interim-results.
- Petrylak DP, Perez RP, Zhang J, et al. A phase I study of enfortumab vedotin (ASG-22CE; ASG-22ME): Updated analysis of patients with metastatic urothelial cancer. J Clin Oncol. 2017;35:15(suppl; abstr 106). ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.106.
- Petrylak DP, Smith DC, Flaig TW, et al. Enfortumab vedotin (EV) in patients (Pts) with metastatic urothelial carcinoma (mUC) with prior checkpoint inhibitor (CPI) failure: A prospective cohort of an ongoing phase 1 study. J Clin Oncol. 2018;36:6(suppl; abstr 431). ascopubs.org/doi/abs/10.1200/JCO.2018.36.6_suppl.431.
- Nadal RM, Mortazavi A, Stein M, et al. Results of phase I plus expansion cohorts of cabozantinib (Cabo) plus nivolumab (Nivo) and CaboNivo plus ipilimumab (Ipi) in patients (pts) with with metastatic urothelial carcinoma (mUC) and other genitourinary (GU) malignancies. J Clinical Oncol. 2018;36:6(suppl; abstr 515). ascopubs.org/doi/abs/10.1200/JCO.2018.36.6_suppl.515.
- Zhang T, Harrison M, O'Donnell P, et al. Phase 2 study of pembrolizumab alone or combined with acalabrutinib in platinum-refractory metastatic urothelial carcinoma (mUC). Presented at: ESMO 2017 Congress; September 8-12, 2017; Madrid, Spain. Abstract 863P. oncologypro.esmo.org/Meeting-Resources/ESMO-2017-Congress/Phase-2-study-of-pembrolizumab-alone-or-combined-with-acalabrutinib-in-platinum-refractory-metastatic-urothelial-carcinoma-mUC.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More