Optimizing Adjuvant Strategies Extends RFS in High-Risk Melanoma
In his presentation at the 2019 Annual Practical Recommendations in Immuno and Molecular Oncology Meetingi, Sanjiv S. Agarwala, MD, reviewed survival statistics of patients with melanoma being treated at different stages of their disease.
Sanjiv S. Agarwala, MD
Current treatment paradigms for melanoma indicate that most patients with node-positive, stage III disease will go on to receive adjuvant therapy with either antiPD-1 immunotherapy or targeted BRAF/MEK inhibitor combinations, according to a presentation by Sanjiv S. Agarwala, MD, of St. Luke’s Cancer Center in Bethlehem, Pennsylvania.1
In his presentation at the 2019 Annual Practical Recommendations in Immuno and Molecular Oncology Meeting in Maui, Hawaii, Agarwala reviewed survival statistics of patients with melanoma being treated at different stages of their disease. Patients with distant metastases have 5-year survival rates of 22.5% versus 63.6% in patients with regional, node-positive disease.2However, patients with stage II/III disease still account for a significant proportion of melanoma-related deaths in the United States.
The once-favored interferon (IFN)-alfa regimens for adjuvant therapy suffered from conflicting efficacy data, controversy over dosage schedules,3and a high toxicity burden. This led IFN-alfa to be surpassed by newer immunotherapy and targeted agents that have moved to the forefront.
AntiCTLA-4: First on the Scene
Ipilimumab (Yervoy) was the first successful checkpoint inhibitor to be used in advanced melanoma. In the randomized, phase III EORTC 18071 trial, ipilimumab induction therapy followed by maintenance with ipilimumab was compared with placebo in treatment-naïve patients with stage III disease following surgical resection. At a median follow-up of 5.3 years, the rate of recurrence-free survival (RFS) at 5 years was 40.8% in the ipilimumab group versus 30.3% with placebo (HR, 0.76; 95% CI, 0.64-0.89;P<.001).4
Despite this, ipilimumab had significant toxicities. Almost all patients (98.7%) experienced adverse effect (AEs) of any grade and about half of patients (54.1%) had grade 3/4 AEs, leading to treatment discontinuation in 53.3% of patients.
AntiPD-1 Therapy Without Gene Mutations
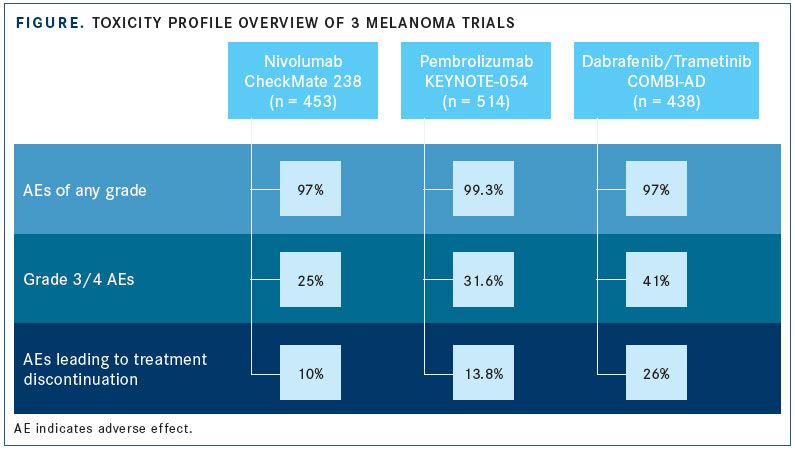
Nivolumab (Opdivo) monotherapy is approved for multiple indications in melanoma, including the adjuvant treatment of node-positive disease, based on results of the phase III CheckMate 238 trial. The trial compared adjuvant therapy of ipilimumab with nivolumab in patients who underwent complete resection of stage IIIB/C or IV melanoma.5Study investigators found that nivolumab improved median RFS over ipilimumab by 6.7 months (P<.0001) and resulted in fewer AEs of any grade. Treatment-emergent AEs led to discontinuation in 10% of patients in the nivolumab arm compared with 43% of patients in the ipilimumab arm.
Similarly, promising results were observed in the phase III EORTC 1325-MG/KEYNOTE-054 trial with the use of pembrolizumab (Keytruda) in patients with completely resected stage III melanoma.6The rate of 1-year RFS in the experimental arm was 75.4% versus 61.0% in the placebo arm (HR, 0.57; 98.4% CI, 0.43-0.74;P<.001). Grade ≥3 AEs occurred in 31.6% of patients taking pembrolizumab versus 18.5% of patients receiving placebo. These data prompted an expanded FDA approval in February 2019 (see page 6).
Targeting the MAPK Pathway
Roughly 40% to 50% of all melanomas have mutations in theBRAFgene and respond well to BRAF/MEK inhibitor combination regimens.1,7The combination of dabrafenib (Tafinlar) plus trametinib (Mekinist) has been approved for the treatment ofBRAF-mutated metastatic melanoma since 2014, but an approval in April 2018 has brought it to the adjuvant setting following surgical resection.
In the phase III COMBI-AD trial, dabrafenib plus trametinib was compared with placebo in patients with stage III disease who harboredBRAFV600E or V600K mutations following complete resection.8The estimated rate of 3-year RFS was improved by 19% with the combination over the placebo (HR, 0.47; 95% CI, 0.39-0.58;P<.001), and the toxicity profile was comparable to earlier trials.
Deciding Between Immune and Targeted Therapy
When deciding between immunotherapy and BRAF/MEK targeted therapy, physicians need to consider the therapies’ toxicity profiles. Toxicities from combination therapy with dabrafenib and trametinib are manageable and can be relieved if treatment is delayed or discontinued; conversely, AEs with antiPD-1 therapy occur at lower incidences but can be more severe and may not resolve with a treatment delay. Based on the information from the previous 3 trials, AEs with dabrafenib plus trametinib led to the most treatment discontinuations (FIGURE).
The combination of nivolumab plus ipilimumab is being studied in patients with stage III melanoma and may result in more treatment options for this patient population (NCT02437279). Other areas of research include the use of these therapies in patients with high-risk stage II disease and as neoadjuvant therapy in resected stage IV disease.
References:
- Agarwala SS. Melanoma: a new paradigm in adjuvant therapy. Presented at: 2019 Annual Practical Recommendations in Immuno and Molecular Oncology Meeting; January 30-February 2, 2019; Maui, HI.
- Cancer stat facts: melanoma of the skin. National Cancer Institute website. seer.cancer.gov/statfacts/html/melan.html. Accessed February 14, 2019.
- Ives NJ, Suciu S, Eggermont AMM, et al; International Melanoma Meta-Analysis Collaborative Group (IMMCG). Adjuvant interferon- for the treatment of high-risk melanoma: an individual patient data meta-analysis. Eur J Cancer. 2017;82:171-183. doi: 10.1016/j.ejca.2017.06.006.
- Eggermont AMM, Chiario-Sileni V, Grob J-J, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy.N Engl J Med.2016;375(19):1845-1855. doi: 10.1056/NEJMoa1611299.
- Weber JS, Mandalà M, Del Vecchio M, et al. Adjuvant therapy with nivolumab (NIVO) versus ipilimumab (IPI) after complete resection of stage III/IV melanoma: updated results from a phase III trial (CheckMate 238). Presented at: American Society of Clinical Oncology 2018 Annual Meeting; June 4, 2018; Chicago, IL. ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.9502.
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma.N Engl J Med. 2018;378(19):1789-1801. doi: 10.1056/NEJMoa1802357.
- Targeted therapy for melanoma skin cancer. American Cancer Society website. cancer.org/cancer/melanoma-skin-cancer/treating/targeted-therapy.html. Updated June 28, 2018. Accessed February 14, 2019.
- Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage IIIBRAF-mutated melanoma.N Engl J Med.2017;377(19):1813-1823. doi: 10.1056/NEJMoa1708539.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More