Unique Combinations Overtake Chemotherapy in Metastatic UC
Optimal therapy in the treatment of metastatic urothelial cancer has evolved over time with regulatory changes pulling back frontline indications.
Matthew Galsky, MD

Optimal therapy in the treatment of metastatic urothelial cancer has evolved over time with regulatory changes pulling back frontline indications. This prompted further research to develop unique combinations that involve immune checkpoint inhibitors and antibody-drug conjugates, which may eventually overtake chemotherapy.
“As the field matures, it’s important to consider that we may no longer have 1 standard of care, but will need to have multiple standards of care,” said Matthew Galsky, MD, during a presentation at the 12th European Multidisciplinary Congress on Urological Cancers (EMUC20), held virtually. Galsky, a professor of medicine, hematology, and medical oncology at Icahn School of Medicine at Mount Sinai in New York, New York, provided his perspective on recent trials that have informed current standards of treatment.
Historically, cisplatin-based chemotherapy was the standard approach in the late 1980s. Over the ensuing years, treatment regimens became more tolerable and were refined for patients, particularly for those who were considered cisplatin eligible. The usual regimen involved treatment with a fixed duration of platinum-based chemotherapy, typically 6 cycles in the absence of disease progression, and then discontinuation of treatment and monitoring. This discontinuation was not because of durable responses, but because the toxicities associated with the regimen accumulated and the benefits would soon plateau.
First-Line Treatment in Metastatic Urothelial Cancer
Two recent trials have arguably had the largest impact on first-line treatment of metastatic urothelial cancer: HCRN GU14-182 (NCT02500121) and JAVELIN Bladder 100 (NCT02603432). These randomized trials incorporated a switch maintenance approach with immune check-point blockade.
HCRN GU14-182 is a randomized phase 2 trial comparing pembrolizumab (Keytruda) with placebo and has a primary end point of progression-free survival (PFS). JAVELIN Bladder 100 is a randomized phase 3 study comparing avelumab (Bavencio) with best supportive care with coprimary end points of PFS and overall survival (OS).
Subsequent trials, such as IMvigor130 (NCT02807636) and KEYNOTE-361 (NCT02853305), are seeking to inform the role of chemotherapy plus immune checkpoint blockade, Galsky said. The second major category of trials evaluated the use of single-versus double-agent checkpoint blockade versus standard platinum-based chemotherapy, and includes DANUBE (NCT02000947) and CheckMate 901 (NCT03036098).
In IMvigor130 and KEYNOTE-361, the addition of immune checkpoint blockade to chemotherapy improved the objective response rate (ORR) in the intention-to-treat population. Specifically, in IMvigor130 the ORR was 47% for the combination of immune check-point blockade plus chemotherapy versus 44% in the chemotherapy-alone arm. Similarly, in KEYNOTE-361, the combination arm of immune checkpoint blockade plus chemotherapy had a 55% ORR versus 45% in the chemotherapy-alone arm.2,3
“Both of these trials included coprimary end points of PFS and OS in the chemotherapy plus immune checkpoint blockade versus chemotherapy-alone populations,” Galsky, who is also codirector of the Center of Excellence for Bladder Cancer at The Tisch Cancer Institute and the Icahn School of Medicine, said. He noted that IMvigor130 met its primary PFS end point, whereas KEYNOTE-361 did not, but “the outcomes of these trials were more similar than different and the differences were probably based on trial design.”
Single-agent PD-1/PD-L1
DANUBE, IMvigor130, and KEYNOTE-361 are all aiming to evaluate the efficacy of single-agent PD-1/PD-L1 versus chemotherapy, he said. When reviewing OS, Galsky noted an initial advantage for chemotherapy but that Kaplan-Meier curves cross at about 10 to 12 months.
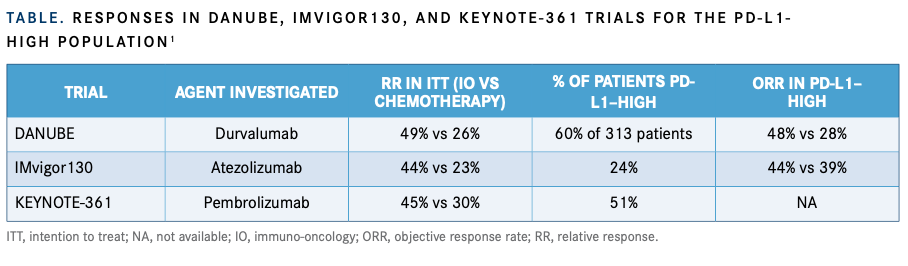
For all these studies, in the intention-to-treat population, the ORR favors chemotherapy ver-sus single-agent immune checkpoint blockade. When looking at the PD-L1–high population in the studies, “the proportion of patients who are deemed PD-L1–high differs significantly across the studies from 24% in IMvigor130 to 60% in DANUBE,” Galsky said (TABLE1).
“In the PD-L1–high population, the ORR doesn’t seem to be enriched that much in the DANUBE study with PD-L1 testing, but in the IMvigor130 study, you do see an enrichment in the ORR. Those results have not been reported in KEYNOTE-361,” Galsky said.
These disparate results in ORR are also seen in the overall survival analyses. There was no improvement in OS with single-agent checkpoint blockade versus chemotherapy in patients with PD-L1–high tumors in DANUBE or KEYNOTE-361.
It should be noted that these studies were not designed to formally test the hypothesis regard-ing single-agent immune checkpoint blockade versus carboplatin-based chemotherapy in patients who are cisplatin ineligible. There have been no exploratory analyses in these subsets of patients to better understand whether or not first-line treatment of patients with PD-L1–high tumors who are cisplatin ineligible should be treated with chemotherapy versus immune checkpoint blockade.
Combinations Involving Immune Checkpoint Blockade Agents
DANUBE evaluated the combination of PD-1/PD-L1 plus CTLA-4 blockade, in which the coprimary end point was OS with durvalumab (Imfinzi) plus tremelimumab versus chemotherapy in the intention-to-treat population.4 Compared with single-agent checkpoint blockade, the use of double-agent immune checkpoint blockade did result in more dramatic Kaplan-Meier survival curves, Galsky said. These results were even more pronounced in the PD-L1–high population, with a benefit seen in the doublet arm.
Checkpoint Blockade Agents Plus Antibody-Drug Conjugates
Galsky is excited about the unique combination involving pembrolizumab and enfortumab vedotin (Padcev) in the EV-103 trial (NCT03288545). Findings presented at the 2020 Genitourinary Cancers Symposium noted an ORR of 73% in previously untreated patients with locally advanced or metastatic urothelial cancer who were ineligible for cisplatin-based chemotherapy.5 The manufacturer said that discussions with the FDA could potentially support registration under accelerated approval regulations.6
“This combination and others will continue to be explored in ongoing trials. Whether or not this regimen will displace platinum-based chemotherapy as a first-line treatment is eagerly awaited,” concluded Galsky.
REFERENCES:
1. Galsky M. Optimal first line therapy in metastatic disease. Presented at: 12th European Multidisciplinary Congress on Urological Cancers. November 13-14, 2020; Virtual. Accessed December 17, 2020. https://bit.ly/37pvKIG
2. Galsky MD, Arija JÁA, Bamias A, et al; IMvigor130 Study Group. Atezolizumab with or without chemotherapy in metastatic urothelial can-cer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395(10236):1547-1557. doi:10.1016/S0140-6736(20)30230-0
3. Alva A, Csőszi T, Ozguroglu M, et al. Pembrolizumab (P) combined with chemotherapy (C) vs C alone as first-line (1L) therapy for advanced urothe-lial carcinoma (UC): KEYNOTE-361. Ann Oncol. 2020;31(suppl 4):S1155. doi:10.1016/annonc/annonc325
4. Powles T, van der Heijden MS, Castellano D, et al; DANUBE Study In-vestigators. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020;21(12):1574-1588. doi:10.1016/S1470-2045(20)30541-6
5. Rosenberg JE, Flaig TW, Friedlander TW, et al. Study EV-103: prelimi-nary durability results of enfortumab vedotin plus pembrolizumab for local-ly advanced or metastatic urothelial carcinoma. J Clin Oncol. 2020;38(sup-pl 6):441. doi:10.1200/JCO.2020.38.6_suppl.441
6. Seattle Genetics announces potential accelerated approval pathway in the U.S. for Padcev™ (enfortumab vedotin-ejfv) in combination with im-mune therapy pembrolizumab as first-line treatment for advanced urothe-lial cancer. Seattle Genetics, Inc. April 02, 2020. Accessed January 10, 2021. https://bit.ly/39lyy9A

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More