Tyrosine Kinase Inhibitors for the Treatment of Chronic Myeloid Leukemia
Chronic myeloid leukemia is a rare type of cancer, and tyrosine kinase inhibitors have become the standard guideline-recommended treatment for patients with <em>BCR-ABL1</em>–positive or Ph-positive CML.
1The median age at diagnosis is approximately 64 years, with the incidence higher in men than in women.2CML occurs in 3 phaseschronic, accelerated, and blast—with most diagnoses reported in the chronic phase.3
CML is characterized by a translocation event t(9;22)(q34;q11.2), in which the Abelson murine leukemia viral oncogene homolog 1 (ABL1) gene is rearranged with breakpoint cluster region(BCR)genes, yielding aBCR-ABL1fusion gene.4The abnormal gene product of thisBCR-ABL1rearrangement is a tyrosine kinase that is constitutively active, causing dysregulation of cell proliferation. The translocation, or rearrangement, of chromosomes 9 and 22 is the most common chromosomal abnormality observed among patients with CML, resulting in the Philadelphia (Ph) chromosome.3
In a recent video interview withTargeted Therapies in Oncology(available at targetedonc.com/precision-medicine/topic/chronic-myeloid-leukemia), Jorge Cortes, MD, deputy chair and professor of medicine in the Department of Leukemia at The University of Texas MD Anderson Cancer Center, noted, “the one thing that’s unique in CML is that, by definition, every patient with the disease has the same molecular abnormalitiesthat is, a rearrangement betweenBCRandABL1which is manifested usually with the Ph chromosome. That’s a cytogenetic manifestation of that molecular rearrangement.”
Tyrosine kinase inhibitors (TKIs) are oral targeted agents that have become the standard guideline-recommended treatment for patients withBCR-ABL1positive or Ph-positive CML.3The aim of oral TKI treatment is to obtain optimal hematologic, cytogenetic, and molecular responses ([MRs] typically measured as ratios ofBCR-ABL1toABL1, which are determined by assessments of peripheral blood). Clinical evidence supports the use of imatinib (a first-generation TKI); dasatinib, nilotinib, and bosutinib (second-generation TKIs); and ponatinib (a third-generation TKI) for the treatment of CML.5-8According to Cortes, however, “there is a variability in terms of the profiles of kinases that are inhibited.” Differences in chemical structures among first-, second-, and third-generation agents lead to slight nuances in mechanisms of action and adverse effect (AE) profiles.
Although TKI therapy has changed the landscape for the treatment of CML, researchers and clinicians have learned that over time, some patients develop resistance or an intolerance to therapy. In fact, more than 25% of patients with CML will switch TKI treatments at least once during their lifetime because of intolerance or resistance.9This article reviews the current TKI landscape for the treatment of CML, examines the implications of resistance, and explores the challenges and opportunities associated with TKI therapy.
FIRST- AND SECOND-GENERATION TKIs
The first TKI for the treatment of CML, imatinib, was approved by the FDA in 2001. Since then, several TKIs have entered the market; the long-term efficacy and safety have been further investigated and elucidated.
First-Generation TKIs
Imatinib.Imatinib is a guideline-recommended first-line TKI treatment option for patients with Ph+ CML in chronic phase (CML-CP).3,10Imatinib was the first signal transduction inhibitor to be used in a clinical setting.11
The International Randomized Study of Interferon and STI571 (IRIS) trial was a prospective, multicenter, open-label, phase III, randomized controlled study that compared imatinib 400 mg once daily (n = 553) with interferon alpha (IFN-α) plus low-dose cytarabine (n = 553) in newly diagnosed (≤6 months prior to study entry) adults aged 18 to 70 with Ph+ CML-CP.12Results of the study provided strong evidence that imatinib was superior to treatment with IFN-α plus cytarabine. At 18 months, rates of major cytogenetic response (MCyR) and complete cytogenetic response (CCyR) were both significantly higher in patients treated with imatinib versus those treated with IFN-α plus cytarabine (87.1% vs 34.7%, respectively;P<.001 and 76.2% vs 14.5%, respectively.12Estimated rates of freedom from progression to accelerated phase (AP) or blast crisis (BC) at 12 months also were significantly higher in the imatinib group than in the combination-therapy group (98.5% vs 93.1%, respectively;P<.001).12In addition, the IRIS trial demonstrated that imatinib was better tolerated than IFN-α plus cytarabine, with more patients in the IFN-α-plus-cytarabine group experiencing AEs of a higher severity (grade 3 or grade 4); AEs experienced by patients in the imatinib group tended to be milder (grade 1 or grade 2). AEs led to a discontinuation of imatinib in 4% of patients. Although the overall survival (OS) rate at 18 months in the imatinib treatment arm was higher than that in the IFN-α-plus-cytarabine treatment arm, it did not reach statistical significance (97.2% vs 95.1%, respectively;P= .16).12
At 8 years, 55% (304 of 553) of patients who had started imatinib in the IRIS trial had continued taking the drug.7The remaining 45% of imatinib-treated patients had discontinued therapy because of AEs or safety issues (6%), unsatisfactory therapeutic outcome (16%), receipt of a stem cell transplantation (3%), death (3%), or other reasons such as failure to renew consent (17%).7Freedom from progression to AP or BC at 8 years among those who had continued taking imatinib was 92%, and OS was estimated at 85%. In a subset of patients whose MRs were monitored sequentially (n = 98), imatinib provided durable responses (24% major molecular response [MMR] at 6 months, 39% MMR at 12 months, and 86% MMR at 8 years), with none of the patients who had documented MMR progressing to AP or BC at 12 months.7These data were pivotal in establishing imatinib as a frontline agent for patients with newly diagnosed CML.
Second-Generation TKIs
Dasatinib.Dasatinib is a second-generation TKI that was approved by the FDA in 2006 for the treatment of imatinib-resistant or imatinib-intolerant adults with Ph+ acute lymphoblastic leukemia (ALL) or Ph+ CML in the chronic, accelerated, or blast phase.13It can also be used as a frontline treatment for patients with newly diagnosed CML-CP.
The DASISION trial was an open-label, multinational, randomized phase III trial that evaluated the safety and efficacy of dasatinib.6Patients with newly diagnosed CML-CP were randomized to receive either dasatinib 100 mg once daily or imatinib 400 mg once daily. Dasatinib demonstrated superior efficacy compared with imatinib at both 12 and 24 months; safety profiles were similar between the 2 agents.6Patients’ responses to dasatinib were both significantly deeper and faster compared with imatinib; 2 other trials also confirmed these findings.14,15
Nilotinib.Approved in 2007 by the FDA, nilotinib is another second-generation TKI for the treatment of imatinib-resistant or imatinib-intolerant patients with Ph+ CML.16The agent is also approved for use as a frontline treatment option in patients with newly diagnosed CML-CP.16
The ENESTfreedom trial investigated the efficacy of nilotinib as a frontline treatment in patients with CML. Treatment free remission (TFR) was demonstrated 12 months following frontline nilotinib therapy. Patients were enrolled if they had Ph+ CML-CP with an MMR and >2 years of frontline therapy with nilotinib. Of the 215 patients who entered the consolidation phase, 190 moved onto the TFR phase. The median duration of nilotinib before treatment was stopped was 43.5 months.5
In 2019, results from 192-week data from the ENESTfreedom trial were published, showing a TFR rate of 44.2% (95% CI, 37.0%-51.6%) and a treatment-free survival rate of 48.7% (95% CI, 41.4%-55.6%) in patients who received nilotinib.17
Bosutinib.Bosutinib is a second-generation TKI currently used to treat patients with CML after the failure of or intolerance to ≥2 other TKIs.18The agent can also be prescribed if after the failure of the first TKI therapy, another option is not practical.19Bosutinib was granted accelerated approval by the FDA in 2017.20The agent has been shown to be capable of overcoming most imatinib-resistantBCR-ABL1mutations.19
The ongoing, multinational, randomized, phase III Bosutinib Trial in First-Line Chronic Myelogenous Leukemia Treatment (BFORE) trial demonstrated the efficacy and safety of bosutinib in patients with newly diagnosed CML-CP. A total of 536 patients were randomized to receive either bosutinib 400 mg once daily (n = 268) or imatinib (n = 268).8Patients treated with bosutinib, compared with imatinib-treated patients, achieved responses faster and had significantly higher rates of MMR (47.2% vs 36.9%, respectively;P= .02) and CCyR (77.2% vs 66.4%, respectively;P= .0075).8Overall, 1.6% and 2.5% of patients who received bosutinib and imatinib, respectively, experienced disease progression to AP/BC. A higher rate of treatment discontinuation was observed in the imatinib group (26.8%) than in the bosutinib group (22.0%), most commonly due to drug-related toxicity (12.7% and 8.7%, respectively).8AEs that were more common with bosutinib than with imatinib included grade ≥3 diarrhea (7.8% vs 0.8%, respectively); increased alanine aminotransferase levels (19.0% vs 1.5%, respectively); and increased aspartate aminotransferase levels (9.7% vs 1.9%, respectively).8The occurrence of cardiovascular (CV) toxicities was uncommon.8
Overview of Treatment Selection
Patient factors to consider when selecting a therapy may include comorbidities, concomitant medications, lifestyle, risk factors,BCR-ABL1transcript type, and additional chromosomal abnormalities.21Broadly, regarding second-generation TKIs, Cortes noted that responses are generally greater compared with responses with the first-generation TKI imatinib, albeit in varying proportions. “With second-generation tyrosine kinase inhibitors, you get deeper responses, you get faster responses; you actually get more responses and a lower rate of transformation,” he indicated. “They don’t give you a survival advantageor at least they haven’t so far—and they don’t give you a progression-free survival [PFS]…an event-free survival. But that’s probably not that unrealistic, considering that with imatinib, you do get a high cytogenetic response rate and with that, the survival already goes pretty close to the normal life expectancy.” Cortes also observed that data are generally comparable among the second-generation agents: “There’s no direct comparison between the 3 drugs, but if you put the studies side by side, it’s very hard to make too much out of those small differences.”
SECONDARY RESISTANCE AND THIRD-GENERATION TYROSINE KINASE INHIBITORS
Amid the development and approval of first-generation and second-generation TKIs for the treatment of CML, treatment resistance as a result ofBCR-ABLkinase domain mutations has emerged as a significant challenge to achieving optimal outcomes. Two forms of resistance to TKIs are recognized: (1)primary resistance, which is defined as a failure to achieve a landmark response, and (2)secondary resistance, which is defined as the achievement and subsequent loss of a response.22Secondary resistance via treatment-emergent mutations has been shown to be strongly predictive of treatment failure.14Given the frequency of resistance that occurs with TKI therapy, patients are regularly monitored after TKI treatment initiation in the chronic phase, in order to identify risks for suboptimal response or treatment failure
(Figure).23,24
According to 8-year follow-up data of the first-generation TKI imatinib, treatment with the agent fails in up to 40% of cases, some of which can be attributed to AEs but many of which are the result ofBCR-ABLkinase domain mutations.7Threonine-to-isoleucine mutation at position 315 (T315I) is perhaps the most common mutation of theBCR-ABLkinase domain, affecting approximately 20% of patients with resistant or relapsed CML.24Patients who move on to a second-generation TKI may experience a response, but 37% to 52% of patients do not, and 23% to 26% of patients who experience an initial MCyR do not retain it after 2 years. The prognosis for these patients is very poor.25
Ponatinib
According to Cortes, the discovery of the T315I mutation led to the development of ponatinib, which, he noted, is perhaps the most potent of all the TKIs. “It covers all the mutations that we know can develop in patients with resistance to TKIs,” Cortes explained. Ponatinib inhibits both unmutated and mutatedBCR-ABL, particularly CML with the T315I mutation.25It is indicated for adult patients with refractory CML or Ph+ ALL and in those with theBCR-ABL1T315I mutation.26
The open-label, multinational, phase II Ponatinib Ph- positive Acute Lymphoblastic leukemia [ALL] and CML Evaluation (PACE) clinical trial evaluated the efficacy and safety of ponatinib.27A total of 449 patients with CML in any phase (chronic phase, AP, or BC) or Ph+ ALL were enrolled in the study.27To be eligible, patients had to have experienced resistance or intolerance to either dasatinib or nilotinib or to have developed the T315I mutation following any TKI therapy. Enrolled patients received ponatinib at a starting dose of 45 mg once daily.27SeeTable17,27-29for a list of clinical trial outcomes in patients with an inadequate response to TKIs in the first line, including second-generation agents.
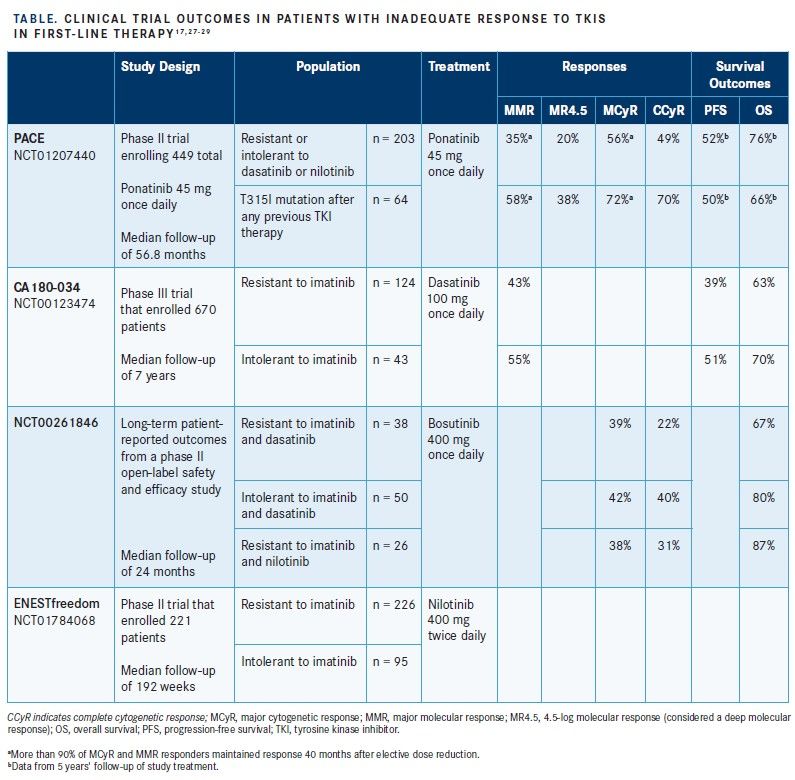
Among 267 patients with CML-CP, 64 of whom had the T315I mutation, 56% had obtained MCyR, 46% had reached CCyR, 34% had achieved MMR, and 15% experienced a deep MR, defined as an MR with a 4.5 log reduction (MR4.5)4after 2 months of ponatinib therapy.25The results were particularly striking among patients with CML-CP who had the T315I mutation. Of these patients, 70%, 66%, 56%, and 23% had achieved MCyR, CCyR, MMR, and MR4.5, respectively. Among all patients in the study with CML-CP, PFS and OS at 12 months were estimated to be 80% and 95%, respectively, using Kaplan-Meier methods for censoring.25The most common serious AE among all study participants (CML-CP, CML-AP, and CML-BC) was pancreatitis (6.4%). Common hematologic AEs included thrombocytopenia (37.2%), neutropenia (18.7%), and anemia (13.4%). Serious CV, cerebrovascular, and peripheral vascular AEs related to treatment with ponatinib were observed in 2.0%, 0.4%, and 0.4% of patients, respectively.25Overall, 12% of all study participants (CML-CP, CML-AP, CML-BC, and Ph+ ALL) discontinued treatment because of an AE, with the most common reason being thrombocytopenia in 4.0% of all study
participants.
At the 5-year mark of the PACE trial, the disposition among the CML-CP enrollees was as follows: 60% with MCyR, 54% with CCyR, 40% with MMR, and 24% with MR4.5.27Among the subset of patients with CML-CP who had the T315I mutation, the disposition at 5 years was 72% with MCyR, 70% with CCyR, 58% with MMR, and 38% with MR4.5.27Kaplan-Meier estimates of ponatinib treatment durability suggested that 82% of patients with CML-CP who had achieved MCyR at 12 months would likely have maintained that response at 5 years. PFS and OS rates at 5 years among all CML-CP enrollees were estimated to be 53% and 73%, respectively, with patients who had the T315I mutation exhibiting PFS and OS rates of 50% and 66%, respectively, at 5 years.27
Approximately 2 years into the study (October 2013), the accumulation of arterial occlusive events (AOEs) became a concern, and elective, preemptive dose reductions to 30 mg or 15 mg were implemented for those who remained in the study. Among participants with CML-CP who were in MCyR at the time of a dose reduction, 96% maintained MCyR until the end of study follow-up (February 2017).27Moreover, 90% of patients with CML-CP who were in MMR at the time of a dose reduction maintained MMR until the study end. For comparison, 94% and 95% of patients who did not undergo dose reduction maintained MCyR and MMR, respectively, from October 2013 to February 2017.27
According to Cortes, the PACE results are significant within the broader context of the TKI treatment spectrum: “Sixty percent of patients had received at least 2 TKIs, and 90% [of patients] had received at least 3 TKIs, and yet the weight of major cytogenetic response in the chronic phase was over 65% in these patient populations. [Ponatinib is] incredibly effective if you put that in the context.” In addition to supporting the indication of ponatinib, Cortes remarked that the PACE results suggest ponatinib may have potential for use earlier in the disease. “One area [in which] we have very little data with other drugs…I think that ponatinib is probably the best option…is in second-line [options], where your first-line [option] is a second-generation TKI. If your first-line agent is imatinib, should we have data with dasatinib, erlotinib, and bosutinib, [which] work well in a number of patients? But it may be possible that ponatinib works better than those.”
Post Hoc Safety Analyses of Ponatinib and Head-to-Head Studies.In a post hoc study to investigate the safety of ponatinib, investigators evaluated results from the PACE trial and other studies to further explore the association of ponatinib dose intensity with arterial occlusive disease and other AEs.30Findings revealed that higher ponatinib doses were associated with higher rates of most AEs, with the strongest associations observed with pancreatitis, rash, and cardiac failure. In addition to prior history of ischemic disease and older age, dose was found to be an independent factor that was strongly associated with the development of AOEs.30The researchers noted that these post hoc findings suggest a potential causal relationship between dose intensity and certain AEs. Moreover, starting ponatinib at a lower dose and/or reducing the dose after a response has been achieved may lead to more effective disease management and optimize outcomes in individuals who are treated with the agent.30
Other post hoc studies have investigated the potential of ponatinib versus other CML treatments. One retrospective study compared the OS among T315I-positive patients who received ponatinib in a phase II trial with the OS of patients who underwent allogeneic stem cell transplantation (allo-SCT), a treatment that had been commonly used in patients with CML prior to the approval of TKIs.31Both 24-month and 48-month OS rates were significantly higher in patients with CML-CP who received ponatinib compared with those who underwent allo-SCT (24 months: 84% vs 60.5%, respectively;P= .004; 48 months: 72.7% vs 55.8%, respectively;P= .013).31The investigators concluded that ponatinib represents a valuable alternative to allo-SCT in patients with T315I-positive CP-CML.31
A more recent study compared the benefitrisk profiles of ponatinib versus bosutinib for the third-line treatment of patients with CML-CP.32Researchers performed a matching-adjusted indirect comparison to evaluate the efficacy outcomes and treatment duration after adjusting for patients’ baseline characteristics. They found that ponatinib was associated with more frequent and durable responses. Additionally, at 4 years, ponatinib was associated with a higher CCyR than bosutinib (61% vs 26%, respectively).32Treatment duration was also longer in ponatinib-treated patients compared with bosutinib-treated patients (38.4 months vs 8.6 months, respectively).32Moreover, the treatment discontinuation rate due to death, disease progression, or unsatisfactory response was considerably lower in the ponatinib treatment arm than in the bosutinib treatment arm (9% vs 42%, respectively), whereas the treatment discontinuation rate due to AEs was 19% in ponatinib-treated patients versus 24% in bosutinib-treated patients.32
CONSIDERATIONS FOR TREATMENT: COMORBIDITIES, TOXICITIES, AND ADHERENCE
Despite the documented benefits of TKIs for the treatment of CML, several challenges to ensuring optimal outcomes remain. Patient comorbidities are a significant concern when selecting a treatment.33When choosing a TKI therapy for the treatment of CML, it is important to take long-term toxicities, particularly cardiopulmonary AEs, into consideration. For example, despite the substantial impact of TKIs on the management of CML, evidence increasingly suggests that the use of these agents is associated with CV and metabolic complications.34Additionally, older patients may have comorbidities and risk factors that increase their likelihood of experiencing TKI-mediated toxicities while undergoing long-term treatment.35
Treatment discontinuation may be warranted in certain cases, particularly given the extended duration of TKI administration.36In a 2019 study by Chamoun and colleagues, the medical records of 100 patients with CML who were in MR and discontinued their TKI treatment outside clinical trials were reviewed. Patients were followed after 30 months of TKI discontinuation (range, 5-112 months). Overall, 35% of patients lost MR and 17% lost MMR after TKI discontinuation, whereas just 6% of patients lost MR ≥12 months after TKI discontinuation.36Investigators observed a loss of MR in 29% of patients who had achieved a sustained MR for >2 years prior to discontinuation and in 7% of patients who had achieved a sustained MR duration for >6 years prior to TKI discontinuation.36The findings also revealed that 30% of patients who discontinued while in MR were retreated, with 93% of patients regaining MR (median, 5 months).36
Challenges With Adherence
TKIs require long-term medication adherence to achieve MMR and maintain remission. Obtaining optimal treatment effects with TKIs includes taking the correct dose regularly, at appropriate intervals, and at the right time in relation to meals.37Yet, nonadherence is very common in patients with a chronic disease.37
In a study that evaluated adherence to TKI therapy in patients with CML, investigators conducted a survey of 140 patients 18 years and older who were treated with an oral TKI (imatinib, dasatinib, or nilotinib).37The survey took into consideration gender, age, education, place of residence, family circumstances, and duration of therapy. The study also evaluated whether a relationship existed between how patients perceived their level of adherence to treatment recommendations and how subjectively the required dosage regimen was followed. Results showed that 39% of patients admitted to having skipped at least 1 drug dose during the course of treatment over a 1-month period. Patients also frequently overestimated their own adherence assessment, with approximately 60% of individuals who missed at least 1 drug dose within the last treatment month believing that they were adherent to their treatment recommendations.37The investigators noted that adherence to TKIs may deteriorate over time.37
According to Cortes, even lower rates of nonadherence can have a significant impact on patient outcomes. “Ten percent [of] missed doses in a month means you missed 3 doses. That doesn’t sound [like] that much when you just say, ‘Well, I just missed probably 3 or 4 doses…’ That already reflects a very low probability of getting to these deeper molecular responses, particularly for a patient who wants to think about treatment discontinuation. [A]ll of that makes a difference.” In fact, he emphasized, skipping pills or adjusting doses can affect the way TKI treatment works.
CONCLUSIONS
TKI therapy can help patients with CML achieve a normal life expectancy. Although the use of TKIs has generated great advancements in treatment options, some challenges nonetheless remain, including drug resistance, risk for relapse, and toxicities. Tailoring treatment decisions based on patient characteristics and disease profiles is essential to achieving optimal outcomes. Carefully observing a patient’s response to the selected treatment can help eliminate relapse and resistance. Moreover, optimum patientprovider communication may assist with monitoring for and managing AEs, in addition to potentially curtailing the likelihood of nonadherence. As the TKI treatment armamentarium continues to evolve, the emphasis will focus on overcoming resistance and reducing toxicities.  
REFERENCES
1. National Cancer Institute Surveillance, Epidemiology, and End Results Program (SEER). Cancer stat facts: leukemia chronic myeloid leukemia (CML). National Cancer Institute website. https://seer.cancer.gov/statfacts/html/cmyl.html. Accessed July 31, 2019.
2. What is chronic myeloid leukemia? American Cancer Society website. https://www.cancer.org/cancer/chronic-myeloid-leukemia/about/what-is-cml.html. Updated June 19, 2018. Accessed July 25, 2019.
3. Radich JP, Deininger M, Abboud CN, et al. Chronic myeloid leukemia, version 1.2019, NCCN Clinical Practice Guidelines in Oncology.J Natl Compr Canc Netw.2018;16(9):1108-1135. doi: 10.6004/jnccn.2018.0071.
4. Kim T, Tyndel MS, Kim HJ, et al. Spectrum of somatic mutation dynamics in chronic myeloid leukemia following tyrosine kinase inhibitor therapy.Blood.2017;129(1):38-47.doi: 10.1182/blood-2016-04-708560.
5. Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study.Leukemia. 2017;31(7):1525-1531. doi: 10.1038/leu.2017.63.
6. Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia.N Engl J Med. 2010;362(24):2260-2270. doi: 10.1056/NEJMoa1002315.
7. Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon Vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib.Blood. 2009 114:1126. Abstract 1126.
8. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial.J Clin Oncol. 2018;36(3):231-237. doi: 10.1200/JCO.2017.74.7162.
9. Patel AB, O’Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors.Hematol Oncol Clin North Am. 2017;31(4):589-612. doi: 10.1016/j.hoc.2017.04.007.
10. Gleevec [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/gleevec_tabs.pdf.Accessed August 1, 2019.
11. Sacha T. Imatinib in chronic myeloid leukemia: an overview.Mediterr J Hematol Infect Dis. 2014;6(1):e2014007. doi: 10.4084/MJHID.2014.007.
12. O’Brien SG, Guilhot F, Larson RA, et al; IRIS Investigators. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia.N Engl J Med.2003;348(11):994-1004. doi: 10.1056/NEJMoa022457.
13. Sprycel [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; 2018. http://packageinserts.bms.com/pi/pi_sprycel.pdf. Accessed August 1, 2019.
14. Radich JP, Kopecky KJ, Appelbaum FR, et al. A randomized trial of dasatinib 100 mg versus imatinib 400 mg in newly diagnosed chronic-phase chronic myeloid leukemia.Blood.2012;120(19):3898-3905. doi: 10.1182/blood-2012-02-410688.
15. Hjorth-Hansen H, Stenke L, Söderlund S, et al; Nordic CML Study Group. Dasatinib induces fast and deep responses in newly diagnosed chronic myeloid leukaemia patients in chronic phase: clinical results from a randomised phase-2 study (NordCML006).Eur J Haematol.2015;94(3):243-250. doi: 10.1111/ejh.12423.
16. Tasigna [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tasigna.pdf. Accessed July 25, 2019.
17. Giles FJ, Masszi T, Gômez Casares MT, et al. Treatment-free remission (TFR) following frontline (1L) nilotinib (NIL) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP): 192-week data from the ENESTfreedom study.J Clin Oncol.2019;37(15 suppl): Abstract 7013.
18. Bosulif [prescribing information]. New York, NY: Pfizer Inc; 2019. http://labeling.pfizer.com/ShowLabeling.aspx?id=884. Accessed August 1, 2019.
19. Isfort S, Brümmendorf TH. Bosutinib in chronic myeloid leukemia: patient selection and perspectives.J Blood Med. 2018;9:43-50. doi: 10.2147/JBM.S129821.
20. FDA grants accelerated approval to bosutinib for treatment of newly-diagnosed PH+ CML. FDA website. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-bosutinib-treatment-newly-diagnosed-ph-cml. Published December 19, 2017. Accessed July 25, 2019.
21. Saglio G, Jabbour E. First-line therapy for chronic phase CML: selecting the optimal BCR-ABL1-targeted TKI.Leuk Lymphoma.2018;59(7):1523-1538. doi: 10.1080/10428194.2017.1379074.
22. Jabbour E, Parikh SA, Kantarjian H, Cortes J. Chronic myeloid leukemia: mechanisms of resistance and treatment.Hematol Oncol Clin North Am. 2011;25(5):981-995. doi: 10.1016/j.hoc.2011.09.004.
23. Jabbour E, Saglio G, Hughes TP, Kantarjian H. Suboptimal responses in chronic myeloid leukemia: implications and management strategies.Cancer. 2012;118(5):1181-1191. doi: 10.1002/cncr.26391.
24. Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors.Blood.2007;110(12):4005-4011.
25. Cortes JE, Kim DW, Pinilla-Ibarz J, et al; PACE Investigators. A phase 2 trial of ponatinib in Philadelphia chromosomepositive leukemias.N Engl J Med.2013;369(19):1783-1796. doi: 10.1056/NEJMoa1306494.
26. Iclusig [prescribing information]. Cambridge, MA: Takeda Pharmaceuticals Company Limited; 2018. https://iclusig.com/pi. Accessed August 1, 2019.
27. Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosomepositive leukemia: final 5-year results of the phase 2 PACE trial.Blood.2018;132(4):393-404. doi: 10.1182/blood-2016-09-739086.
28. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034.Am J Hematol. 2016;91(9):869-874. doi: 10.1002/ajh.24423.
29. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib.Am J Hematol. 2016;91(12):1206-1214. doi: 10.1002/ajh.24536.
30. Dorer DJ, Knickerbocker RK, Baccarani M, et al. Impact of dose intensity of ponatinib on selected adverse events: multivariate analyses from a pooled population of clinical trial patients.Leuk Res. 2016;48:84-91. doi: 10.1016/j.leukres.2016.07.007.
31. Nicolini FE, Basak GW, Kim DW, et al. Overall survival with ponatinib versus allogeneic stem cell transplantation in Philadelphia chromosome-positive leukemias with the T315I mutation. Cancer. 2017;123(15):2875-2880. doi: 10.1002/cncr.30558.
32. Levy MY, McGarry LJ, Huang H, et al. Benefits and risks of ponatinib versus bosutinib following treatment failure of two prior tyrosine kinase inhibitors in patients with chronic phase chronic myeloid leukemia: a matching-adjusted indirect comparison.Curr Med Res Opin.2019;35(3):479-487. doi: 10.1080/03007995.2018.1510225.
33. Caldemeyer L, Dugan M, Edwards J, Akard L. Long-term side effects of tyrosine kinase inhibitors in chronic myeloid leukemia.Curr Hematol Malig Rep. 2016;11(2):71-79. doi: 10.1007/s11899-016-0309-2.
34. Aghel N, Delgado DH, Lipton JH. Cardiovascular toxicities of BCR-ABL tyrosine kinase inhibitors in chronic myeloid leukemia: preventive strategies and cardiovascular surveillance.Vasc Health Risk Manag. 2017;13:293-303. doi: 10.2147/VHRM.S108874.
35. Medeiros BC, Possick J, Fradley M. Cardiovascular, pulmonary, and metabolic toxicities complicating tyrosine kinase inhibitor therapy in chronic myeloid leukemia: strategies for monitoring, detecting, and managing.Blood Rev. 2018;32(4):289-299. doi: 10.1016/j.blre.2018.01.004.
36. Chamoun K, Kantarjian H, Atallah R, et al. Tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia: a single-institution experience.J Hematol Oncol. 2019;12(1):1. doi: 10.1186/s13045-018-0686-1.
37. Rychter A, Jerzmanowski P, Holub A, et al. Treatment adherence in chronic myeloid leukaemia patients receiving tyrosine kinase inhibitors.Med Oncol. 2017;34(6):104. doi: 10.1007/s12032-017-0958-6.