Triplet Therapy Is Active and Safe in MSH mCRC
The triplet regimen of nivolumab, ipilimumab, and panitumumab has shown antitumor activity and a consistent safety profile in patients with previously treated metastatic colorectal cancer that is microsatellite stable and KRAS, NRAS, and BRAF wild type, according to findings from the phase 2 LCCC1632 study.
The triplet regimen of nivolumab (Opdivo), ipilimumab (Yervoy), and panitumumab (Vectibix) has shown antitumor activity and a consistent safety profile in patients with previously treated metastatic colorectal cancer (mCRC) that is microsatellite stable (MSS) and KRAS, NRAS, and BRAF wild type, according to findings from the phase 2 LCCC1632 study (NCT03442569).
“The combination of panitumumab, ipilimumab, and nivolumab met its primary end point with a 35% 12-week response rate and demonstrated promising activity in previously treated KRAS, NRAS, and BRAF wild-type MSS mCRC,” said Michael Sangmin Lee, MD, an assistant professor at The University of Texas MD Anderson Cancer Center. Lee delivered his remarks during a presentation at the 2021 Gastrointestinal Cancers Symposium.
The Simon’s 2-stage phase 2 trial enrolled patients with unresectable and/ or metastatic CRC who had KRAS, NRAS, and BRAF wild-type, MSS or mismatch repair proficient (pMMR) disease. Patients also had received 1 to 2 prior treatment regimens, not including an EGFR inhibitor or immune checkpoint inhibitor.
In the study, investigators administered 6 mg/kg IV panitumumab every 2 weeks to patients, with 240 mg IV nivolumab every 2 weeks and 1 mg/kg IV ipilimumab every 6 weeks.
The primary end point was the 12-week response rate and secondary end points included overall response rate (ORR), progression-free survival (PFS), overall survival (OS), and toxicity.
ho were assessed for tolerability; no dose-limiting toxicities were observed in 12 weeks. Following this, 26 patients were included in stage 1, in which 7 responses were required to justify continued enrollment; investigators observed 9 responses in this stage. In stage 2, they enrolled 24 more patients, with 17 responses being required to justify further study.
With an α of 0.10, the study was statistically designed with 80% power to detect an improvement in response rate from a null hypothesis of 22% to an alternative hypothesis of 35%, Lee explained.
Of the total patient population across the stages of the study, the median age was 56 years (range, 36-74). Sixty-six percent of participants were male, 77% were White, 52% had an ECOG performance status of 1, and 50% had left-sided colon tumors.
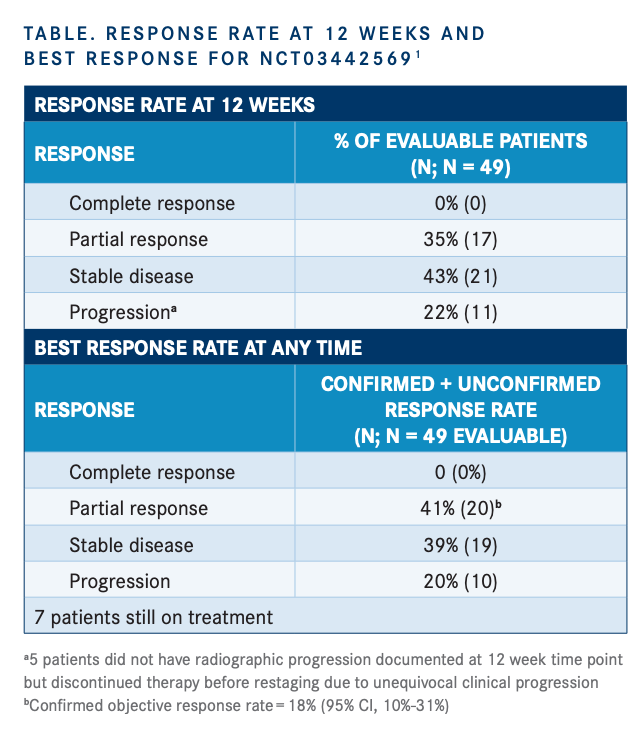
Among 49 patients evaluable for response, the response rate at 12 weeks was 35%, which consisted of all partial responses. Investigators noted that this met the primary end point of the trial. An additional 43% of patients achieved stable disease (SD) and 22% had progression (TABLE).
The ORR was 20% with confirmed responses in 18% (95% CI, 10%-31%) of patients. SD was noted in 39% of patients and disease progression in 20%. Seven patients remained on treatment. A majority of patients achieved a reduction in their tumor size from baseline.
The median PFS was 5.7 months (95% CI, 5.5-7.9) and median OS was 27 months (95% CI, 14.5-not evaluable). However, the OS data are not yet mature.
Common grade 3 to 5 treatment-related adverse events included hypomagnesemia (11%), rash acneiform (11%), lipase increase (9%), and amylase increase (7%). Alanine aminotransferase increase, aspartate aminotransferase increase, diarrhea, hypophosphatemia, and maculopapular rash each occurred in 5% of patients. The 2 grade 5 events included myocarditis, which was possibly related to treatment, and colon perforation, which was not considered likely to be treatment related.
“Though toxicities were of course observed, they were consistent overall with the expected AE profiles of anti-EGFR therapy and combination ipilimumab and nivolumab,” Lee commented.
He noted that correlative studies are ongoing using archival specimens and limited on-treatment biopsies.
REFERENCE:
Lee MS, Loehrer PJ, Imanirad I, et al. Phase II study of ipilimumab, nivolumab, and panitumumab in patients with KRAS/NRAS/BRAF wild-type (WT) microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39(suppl 3):7. doi:10.1200/JCO.2021.39.3_suppl.7
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More