FGFR Inhibitors Make Inroads in Cholangiocarcinoma
Although chemotherapy is the mainstay of systemic treatment for advanced cholangiocarcinoma, there are limited treatment options when the disease progresses
Rachna Shroff, MD

Although chemotherapy is the mainstay of systemic treatment for advanced cholangiocarcinoma (CCA), there are limited treatment options when the disease progresses. Investigators are evaluating multiple studies that are focused on targeted therapies in this setting, and the results appear positive.1
“This is a disease where every single day I feel we’re making meaningful progress, and it’s exciting to be part of this world right now because you can see the forward motion happening,” Rachna Shroff, MD, associate professor in the Department of Medicine, Division of Hematology and Oncology, and chief of gastrointestinal medical oncology at the University of Arizona Cancer Center in Tucson, said in an interview with Targeted Therapies in Oncology.
Tanios S. Bekaii-Saab, MD

Next-generation sequencing (NGS) has identified multiple targetable oncogenes in CCA. Approximately 45% of patients with intrahepatic cholangiocarcinoma (iCCA) have actionable genetic alterations with fibroblast growth factor receptor 2 (FGFR2) fusions or rearrangements occurring exclusively in iCCA.2
“Cholangiocarcinoma is a very target-rich disease. From day 1, we need to understand the genetic landscape of this cancer [and patients should have NGS data],” Tanios S. Bekaii-Saab, MD, professor of medicine and consultant, Division of Hematology/ Oncology at Mayo Clinic in Phoenix, Arizona, said in an interview with Targeted Therapies in Oncology.
FGF/FGFR is a signaling pathway responsible for regulating cell survival and mediating physiological functions. If the FGF/FGFR pathway is disrupted due to genetic alterations, malignancies may arise, including CCA.1 FGFR2 gene fusions or rearrangements are the most common oncogenic drivers in iCCA, accounting for approximately 13% of mutations.1,3 “Although this is a rare tumor with a rare subset, it [comprises] a meaningful percentage of patients in a disease that otherwise has very limited therapeutic options,” Shroff said.
Recently, the development of FGFR inhibitors has provided a promising class of agents in the treatment of CCA, especially since FGFR2 fusion may not respond better to chemotherapy compared with FGFR2 wild type.1,3 Results from a retrospective analysis demonstrated that survival outcomes in patients with iCCA harboring FGFR2 fusions (n = 15) have similar median progression-free survival (PFS) compared with FGFR2 wild type (n = 107), 6.2 months (95% CI, 2.0-16.8) versus 7.2 months (95% CI, 5.0-8.3) for firstline standard chemotherapy treatment, respectively.3
“FGFR fusions and subsequent inhibition with targeted therapy [are] of great interest in cholangiocarcinoma primarily because we have multiple trials as proof of principle that we can effectively target this pathway,” Shroff said.
Three Agents in Development There are currently 3 FGFR inhibitors that treat CCA. Pemigatinib (Pemazyre) is the first FDA-approved agent for CCA based on results from the FIGHT-202 clinical trial (NCT02924376).4 FIGHT-202 is a phase 2, single-arm, multicenter, multicohort trial that evaluated the safety and efficacy of pemigatinib in previously treated, locally advanced, or metastatic CCA.5 In 107 patients evaluated with FGFR2 fusions, the objective response rate (ORR) was 35.5% (95% CI, 26.5%-45.3%). The median duration of response (DOR) was 7.5 months (95% CI, 5.7-14.5) with disease control rate (DCR) of 82.2%. The median PFS was 6.9 months (95% CI, 6.2-9.6), and preliminary median OS was 21.1 months (95% CI, 14.8-not estimable).5
Other FGFR inhibitors evaluated after progression on chemotherapy include infigratinib and futibatinib. Infigratinib was evaluated in advanced CCA in a single-arm, phase 2 trial (NCT02150967) in patients harboring FGFR2 fusions presented at the 2021 Gastrointestinal Cancers Symposium. Approximately 160 patients are to be enrolled in 3 cohorts, with results from cohort 1 (n = 108) presented at the conference. ORR was observed in 23.1% (95% CI, 15.6-32.2). The ORR was higher in the second-line setting compared with third or later lines, 34% versus 13.8%, respectively. The median PFS was 7.3 months (95% CI, 5.6-7.6), and DOR was approximately 5 months (range 0.9-19.1).6
Commenting on the studies with pemigatinib and infigratinib, Shroff said, “More [patients in] the infigratinib patient population have progressed on multiple lines of chemotherapy [compared with] pemigatinib. [Approximately] 60%…received pemigatinib as second-line therapy, so a little earlier than what we saw in the infigratinib data.”
Shroff added, “Interestingly, it seems the earlier use of FGFR inhibitors, in the second line for instance, produces a slightly higher response rate, suggesting that there’s utility in moving this targeted therapy into the earlier [lines of treatment].”
The third agent of interest is futibatinib, a third-generation, irreversible FGFR inhibitor. In the phase 2 FOENIX-CCA2 trial (NCT02052778), futibatinib was evaluated in 103 previously treated patients harboring FGFR2 fusion. Data were included for 67 patients with an observed ORR of 37.3%, DCR of 82.1%, and median DOR of 8.3 months.
Adverse Effects One of the challenges of FGFR inhibitors is the associated toxicities. Adverse effects of various FGFR inhibitors are very similar due to the class effect of FGFR inhibition.1 Bekaii-Saab noted, “The [adverse event] that’s very prominent or common is hyperphosphatemia, which does occur in approximately half of patients, although [there were no severe grade toxicities associated with it]. [Hyperphosphatemia] can be managed with a low phosphate diet. Occasionally we use phosphate binders and rarely we require dose reductions or interruptions.”
Shroff noted, “I think what we’ve learned is that [although] we were aggressive in managing [hyperphosphatemia], clinically it did not produce a lot of major issues.”
Other common associated adverse effects include dermatologic toxicities, diarrhea, decreased appetite, fatigue, and liver dysfunction.1
“Because patients end up staying on [FGFR inhibitors] for some time, the more cumulative toxicities…are more relevant,” Shroff stated. “These include nail bed changes that can be quite disabling and painful to our patients, associated skin changes, dry mucous membrane, and stomatitis. There [are some alopecia and ocular adverse effects].…What we’ve learned over time is that [earlier treatment] and using a multidisciplinary team…[are] going to be important.”
Despite the possible adverse effects, BekaiiSaab noted, “[FGFR inhibitors] are quite safe and [toxicities are] relatively manageable for most patients, if not the overwhelming majority of patients.”
First-Line Setting
There are also several studies evaluating the use of FGFR inhibitors in the first-line treatment of patients with CCA (TABLE8-11). “These frontline studies are very important. I think it is a logical question to see if FGFR inhibitors will be able to replace gemcitabine-cisplatin [chemotherapy],” Shroff noted.
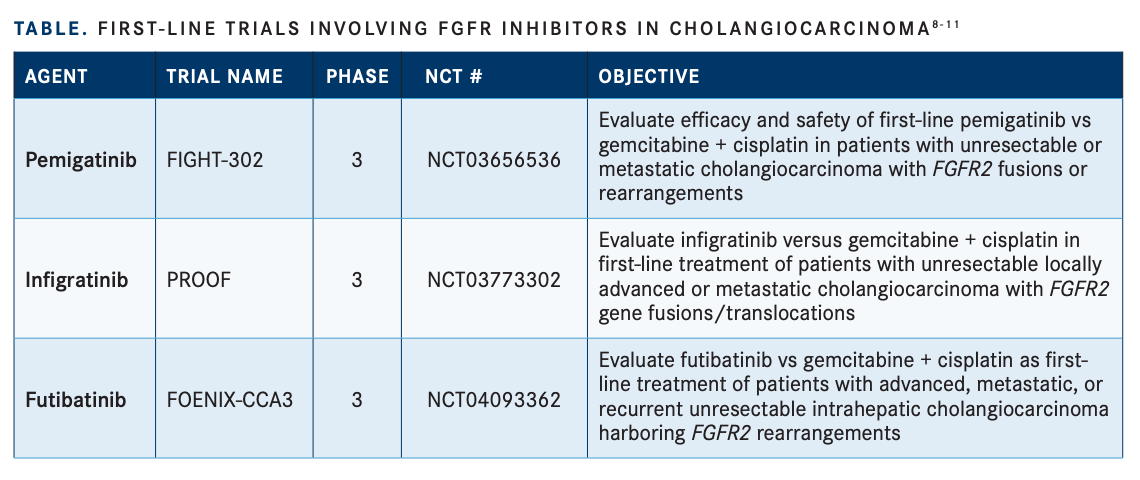
Pemigatinib is being investigated in the first-line treatment of CCA compared with standard of care chemotherapy in the phase 3, randomized, open-label FIGHT-302 study (NCT03656536). Crossover to pemigatinib is permitted upon disease progression from the chemotherapy group.8
“We do think this is probably an even better role for patients with FGFR2 fusion to be exposed to these agents sooner rather than later, but that is what the study [is trying to prove],” noted Bekaii-Saab, who is the study’s principal investigator.
Infigratinib is being evaluated in the firstline setting for advanced CCA compared with chemotherapy in the phase 3 PROOF trial (NCT03773302) with planned enrollment of 384 patients with FGFR2 fusions.9 Additionally, futibatinib will be evaluated in the first-line setting against standard chemotherapy for 216 patients with advanced CCA with FGFR2 fusions in the phase 3, randomized, multicenter, open-label FOENIX-CCA3 trial (NCT04093362).10
“Here’s the good news: a lot of these studies are looking at first-line [use of FGFR inhibitors],” Bekaii-Saab said. “As we keep uncovering more targets, I think we’re going to fill the landscape of cholangiocarcinoma with primarily agents that are targeted specifically for the cancer and move away from chemotherapy, at least in the first-line treatment.”
Acquired Resistance
However, another challenge associated with the use of FGFR inhibitors is acquired resistance, which may develop by mutation activation or if the signaling pathway is bypassed.1 Point mutations in FGFR2 may lead to clinical resistance of FGFR inhibitors in CCA.11
“FGFR2 fusions are early oncogenic drivers, so [they] persist through the course of the patient’s treatment journey. Once [patients] are treated with an FGFR inhibitor, inevitably, they do develop [disease] progression and the presumption is that resistance has developed. There’s some really elegant work that has been done utilizing [circulating tumor DNA] to better understand the mechanism of resistance. What we’ve learned is that resistance is really polyclonal and often involves mutations that change the binding pocket that affects the ability of the FGFR inhibitor to bind, and some of these are what we call gatekeeper mutations,” Shroff said.
There may be differences in mutational resistance due to differing binding specificities of FGFR inhibitors, and there is evidence to suggest polyclonal resistance with multiple acquired FGFR2 mutations in patients treated with infigratinib or pemigatinib in CCA.2,11 Due to the covalent bond of futibatinib to FGFR, there may be clinical benefit in CCA with acquired resistance to FGFR inhibitors. Additionally, strategically sequencing FGFR inhibitors may extend the duration of benefit for FGFR inhibitor use.11
“There’s more to be done since we’re at the tip of the iceberg of trying to understand mechanisms of resistance with these agents,” Bekaii-Saab said. Given the challenges with FGFR inhibitor use, the future of CCA treatment is still extremely promising.
“I would imagine in the next 5 to 10 years, we’re going to continue with this positive upward trajectory and have more drugs available,” Shroff said. “I firmly believe we will move the needle forward in terms of median overall survival for this disease in the advanced setting. I think we will figure out how to integrate some of these novel targeted therapies and [potentially] immunotherapy into more localized disease and perioperative approaches…. At the end of the day, surgery is our curative treatment, and anything we can do to build on outcomes for patients [who] can undergo surgery is obviously very important.”
REFERENCES:
1. Wang J, Xing X, Li Q, et al. Targeting the FGFR signaling pathway in cholangiocarcinoma: promise or delusion? Ther Adv Med Oncol. 2020;12:1758835920940948. doi:10.1177/1758835920940948
2. Silverman IM, Hollebecque A, Friboulet L, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11(2):326-339. doi:10.1158/2159-8290.CD-20-0766
3. Abou-Alfa GK, Bibeau K, Schultz N, et al. Effect of FGFR2 alterations on survival in patients receiving systemic chemotherapy for intrahepatic cholangiocarcinoma. J Clin Oncol. 2021;39(suppl 3):303. doi:10.1200/ JCO.2021.39.3_suppl.303
4. FDA grants accelerated approval to pemigatinib for cholangiocarcinoma with an FGFR2 rearrangement or fusion. FDA. Updated April 20, 2020. Accessed March 5, 2021. https://bit.ly/2QomFdh
5. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671-684. doi:10.1016/S1470-2045(20)30109-1
6. Javle MM, Roychowdhury S, Kelley RK, et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol. 2021;39(suppl 3):265. doi:10.1200/JCO.2021.39.3_suppl.265
7. Goyal L, Meric-Bernstam F, Hollebecque A, et al. FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J Clin Oncol. 2020;38(suppl 15):108. doi:10.1200/ JCO.2020.38.15_suppl.108
8. Goyal L, Shi L, Liu LY, et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9(8):1064-1079. doi:10.1158/2159-8290.CD-19-0182
9. Bekaii-Saab TS, Valle JW, Cutsem EV, et al. FIGHT-302: phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J Clin Oncol. 2020;38(suppl 4):TPS592. doi:10.1200/JCO.2020.38.4_suppl.TPS592
10. Javle MM, Borbath I, Clarke SJ, et al. Infigratinib versus gemcitabine plus cisplatin multicenter, open-label, randomized, phase 3 study in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/ translocations: the PROOF trial. J Clin Oncol. 2019;37(suppl 15):TPS4155. doi:10.1200/JCO.2019.37.15_suppl.TPS4155
11. Borad MJ, Bridgewater JA, Morizane C, et al. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinomva (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J Clin Oncol. 2020;38:(suppl 4):TPS600. doi:10.1200/JCO.2020.38.4_suppl.TPS600