The Emerging Role of Mobile Health in Oncology
This article offers an overview of mobile health technology as it relates to a participatory care model and shared decision making.
Abstract
Advances in wireless technology have given rise to the development of mobile health (mHealth) platforms, also known as telehealth, telemedicine, eHealth, and digital health. These technologies offer a unique opportunity for healthcare providers to remotely deliver high-quality care. The possibilities are endless for the clinical application of mHealth, including tools for payers, decision support, patient support, educational purposes, drug formularies, or medical uses. Moreover, the number of smartphone owners currently using a mobile medical app is expected to increase to 3.4 billion by 2018.1This shift makes patients the consumers of healthcare, empowering them to be the driving force managing their own health through mobile devices and wearable technology. This article will offer an overview of mHealth technology as it relates to a participatory care model and shared decision making, as well as challenges to overcome in clinical practice and research.
The Evolution of Mobile Health Technology
As smartphones replace wallets, online banking, printed books, travel services, CDs, photo albums, and cameras, mobile devices are beginning to reshape many medical functionalities. In view of the fact that 83% of physicians are using smartphones and medical apps to provide patient care, the traditional medical landscape is changing dramatically, with the goal of delivering less expensive, high-quality care.2
Mobile health (mHealth) technology may improve access to specialized medicine, provide more effective preventive care, increase monitoring of chronic conditions, and lead to better patient outcomes. Trends include wireless patient monitoring, personal health record access via patient portals, mobile medical devices, and virtual consultation (telehealth). Health-related applications currently in use include patient communication, access to Internet resources, point-of-care documentation, disease management, educational programs, professional communication, administrative and nancial functions, ambulatory services, public health, and clinical trials.3,4Moreover, the rapid development of mHealth applications has expanded telehealth services to smartphones, tablets, and laptops to deliver precision medicine to patients and families in the comfort of their own home.
mHealth technology allows patients and providers to engage in collaborative partnerships to harness participatory healthcare. For example, a recent study evaluating patient receptiveness to health reminders, media channels, and messengers concluded that half of participants wanted their doctor or nurse to send them a health reminder via text message or phone call, indicating mHealth’s potential to encourage patients to participate in their own care and improve their own health outcomes.5
mHealth Technology in Practice
The technological advances of mHealth include wearable sensors for most physical measures (eg, pedometer/accelerometer, sleep, blood pressure, heart rate, temperature, environment exposure, blood levels, falls, and geolocation), data entry (exercise testing, diet, mood/stress levels, symptoms, health-related quality of life, functional status, social support, medication,tobacco use, and alcohol use),ingestible/ implantable sensors, biometric sticker sensors, and the ability of smartphones to be used as otoscopes, ophthalmoscopes, and microscopes.
Patient apps and sensors can also monitor weight, inhaler use, the cognitive state of the patient, motor impairment, movement, carbohydrate intake, and insulin dose. A single-lead electrocardiogram attached to a smartphone and to a patient’s glucometer can track blood glucose over time, as well as post alerts and help patients to self-manage and keep a log. Aggregated information can be collected outside of clinical encounters and be turned into actionable information using sensors that monitor pillboxes, tremor frequency, vital signs, or imaging/ laboratory tests. These data can then be sent to a healthcare provider (ie, to support drug adherence, reduce medication errors, save time during emergencies, and shape self-management or support clinical decisions in real time).6,7
mHealth interventions and the collection of biometric data have also expanded to social media sites, such as Twitter, to foster healthy lifestyles and facilitate support for behavioral changes.8
The FDA has also approved imaging apps, allowing radiologists to interpret images or ophthalmologists using color vision plates for clinical evaluation when a more traditional viewing platform is not available. Digital images are a type of store-and-forward technology, which permits the electronic transmission of medical files that can be used at the convenience of providers to then make diagnoses and recommendations and formulate treatment plans. As technology advances, devices that provide real-time data monitoring and biometric measurements can give healthcare providers immediate information to aid in clinical decision making.
While still under developmentand requiring evidence to support its useCicer, a monitor that tracks pulse, respiration, blood, and oxygen through the use of predictive algorithms, can stream data to providers. iTBra is currently being developed to help with the early detection of breast cancer without requiring a mammogram. And Aira has developed wearable glasses that stream live videos to agents who provide help with navigation and assistance to patients who are blind or have low vision. These advancements have the potential to revolutionize technology and medicine.
Healthcare Consumers’ and Providers’ Perspectives on mHealth
In a study evaluating healthcare professionals’ opinions on the use of mHealth in oncology, a majority of responders cited several advantages of mHealth apps: better documentation of data and test results, improved and continuous care for patients, enhanced communication between provider and patient, increased patient compliance, and, possibly, scientific evaluation.9Overall, 84.3% of oncology care providers supported the use of apps to complement traditional treatment.9
In another survey of healthcare professionals, the most commonly used medical app functions included drug referencing, clinical decision support, communication, electronic health record (EHR) access, and medical education.10In light of the large amount of medical knowledge clinicians must memorize, reference programs and educational apps enable them to choose clinically appropriate and cost-effective drugs, quickly search and access information/textbooks, perform calculations, log experiences, communicate, and input specific patient information for diagnosis.11Nevertheless, critics cite concerns related to legal uncertainty, data privacy, and insecure data transfer and storage.9
The degree of adoption of mHealth relies on patients’ participation and their ability and motivation to become a partner in their healthcare. Platforms that focus on diseases, therapy, treatment, or symptoms have already redefined health-related social networking and the ability to provide support, information, and advocacy. In addition, increasing out-of-pocket expenses and the current reimbursement structure emphasize a paradigm shift of responsibility to patients to manage their health network and information by leveraging emerging technologies.
In a survey assessing patient attitudes toward mHealth, patients overall had a positive opinion, citing an opportunity for improved self-efficacy and provider-driven medical management.12They highlighted their comfort level with being remotely monitored and having con dence in the privacy protection afforded by mHealth. In addition, findings from cancer survivors’ experiences with mHealth illustrate analytic themes that include how mHealth limited the disruption to their lives and can enable close and personable relationships between cancer survivors and care providers, and how they felt they had immediate access to professional advice that acted as a safety net for possible issues in treatment.13The results of another randomized controlled trial for patients with prostate cancer using mHealth technology after radical prostatectomy show that mHealth is equivalent in patient and provider satisfaction and time allocated to care compared with in-person care.14
Nevertheless, individual differences in digital literacy (ie, having the technical skills to operate digital devices and understand their functionality) have the potential to widen health disparities and must be addressed as mHealth becomes more widely implemented.15,16Research demonstrating the digital disadvantage of individuals of lower socioeconomic status and ethnic minorities has drawn attention to patients who can benefit from the new technology, yet are unlikely to adopt mHealth, as well as individuals who can benefit or lack access to wireless services in remote areas.17
mHealth can help reduce healthcare access disparities in rural areas or among underserved populations that have historically been a challenge due to a shortage of clinicians, nancial means, or geographic barriers. It has been found to increase the quality of care and reduce costs by decreasing hospital readmissions and emergency visits in rural communities18and by providing greater access through clinic attendance in these areas with patient satisfaction up to 94%.19Moreover, in an assessment of mHealth to support outpatient palliative care, telemedicine was used to remotely monitor and manage symptoms of patients with advanced cancer, the technology demonstrated greater access to the healthcare system, reduced the need to employ emergency services, improved assessment/control of symptoms, and provided greater assurance for family members through early interventions.20
Teleoncology has demonstrated effectiveness in the delivery of survivorship care plans in geographic areas where cancer survivors do not otherwise have access and shows potential to increase access to a comprehensive cancer center for patients in rural areas by offering consultations, supervision of chemotherapy administration, oral medication adherence, or symptom management.21-23In an interventional oncology clinical trial using metformin in patients with castration-resistant prostate cancer, telemedicine was proved effective in replacing on-site study visits and showed the potential to overcome barriers in clinical trial participation.24As the lack of access to specialized oncology care increases, teleoncology can also serve as a platform to deliver specialists to underserved areas by bringing a community oncologist and regional specialist together via virtual consultation to streamline care, save time, relieve stress, increase exposure to clinical trials, and reduce disparities in healthcare access.25
mHealth Challenges for Clinical Practice
For all its benefits, the implementation of mHealth technologies remains a challenge in the clinical practice setting. These factors include concerns about privacy, confidentiality, and ownership of data; integration into existing EHR platforms at the healthcare institution or physician office; cultural and organizational barriers that may exist within the practice setting; and regulatory oversight.
Privacy, Security, Confidentiality, and Ownership of Data
For mHealth to complement traditional approaches in the delivery of healthcare, both clinicians and patients must be confident that the privacy, confidentiality, and security of data will be safeguarded in a manner consistent with the Health Insurance Portability and Accountability Act (HIPAA). In a constantly changing field, the means for securing data include understanding the threats to cyber security (ie, viruses, spyware, or hackers) and developing an mHealth technology policy, system, and infrastructure to ensure secured health information (PHI) data are safe and protected against unauthorized use. The challenge with maintaining transparency of data, however, focuses on the question of ownership of that data.
Ownership of patient records has historically been held by the healthcare institution. However, mHealth technology is more complex, giving rise to questions pertaining to which data should be shared and who should be responsible for updating and ensuring data accuracy, and whether there is a clear delineation of roles in monitoring the access, quality, security, privacy, and confidentiality. As mHealth expands to include data collection through smartphones or wearable devices, more opportunities exist for data entry errors, jeopardizing data integrity. Nelson and Staggers26write that “the accuracy and consistency of stored and transmitted data can be compromised when information is en- tered incorrectly or deliberately altered or when the system protections are not working correctly or suddenly fail.” Legal guidance for mHealth technology is needed to ensure privacy and security protection since HIPAA privacy protections may not be applicable to each mHealth application. Thus, usability must be balanced with privacy protection to include an individual’s control over collected data and security protection against inappropriate access or disclosure of information.
EHR Integration
In the progression of mHealth, EHR systems need to establish a method for medical apps and mobile technology to be integrated with existing health IT systems. Patient portals tethered to EHRs can provide scheduling, billing, and clinical support, but there is no policy for mHealth applications to be fully integrated into health information systems in hospitals or provider organizations.26Medical apps and mobile technology that lack the ability to be integrated into EHRs limit the availablility of patient-generated health data that the healthcare team can access.
Application programming interfaces (APIs) offer a potential solution to interoperability problems between mHealth and EHRs by acting as a bridge to manage the open data exchange. An interoperability project at Harvard Medical School and Boston Children’s Hospital, Substitutable Medical Applications and Reusable Technologies on Fast Health Information Resources (SMART onFHIR), was able to establish how “properly designed data and authentication APIs can successfully shield health IT app developers from complexity in integrating with proprietary vendor systems.”27Several provider and patient apps were also successfully integrated using a similar framework, called Epic EHR, which was implemented at Duke Health.28
Adoption/Organizational Culture
Multiple factors on both the individual and organizational levels are crucial to providers’ acceptance and adoption of mHealth technology. Organizations may not be structured to support the patient-centeredness that mHealth technology provides due to the additional time, resources (ie, increased demand for time, longer visits, technical support, effective leadership, provider involvement, or ability to integrate into work flow), management, and educational training needed to engage patients and providers. In order to understand the value of mHealth to patients, providers may need to learn new skills or acquire knowledge through additional training.16Moreover, the economics of healthcare and lack of research regarding which patients would benefit the most, the efficiency of mHealth at cost cutting/time saving, and the effects on provider burden may hinder the advancement of mHealth.
Provider acceptance of mHealth technology depends on a full integration into work flow, added value to patient care, administrative convenience, and facilitated communication among multidisciplinary teams.29Although usefulness and ease of use were cited as important factors to the adoption of mHealth, the question of whether it is an efficient and cost-cutting option is still in discussion among healthcare professionals who have referenced cost issues as limiting its adoption.30,31
Elements related to costs (eg,the question of how to bill for mHealth) and lack of understanding of liability and malpractice serve as further barriers. Furthermore, despite the benefits associated with mHealth technology, safety concerns regarding the quality and accuracy of medical apps and wearables has not been addressed, as developers may not involve individuals with medical expertise in the creation of mHealth technology. With only FDA guidance available, mHealth technology has yet to be subjected to stringent assessment before use.
Regulatory Oversight
On September 24, 2015, the FDA released final guidance on mobile medical applications. “The guidance provides [the] industry with an in-depth explanation of the agency’s current thinking on the appropriate regulation of mobile apps, limiting its scope to apps that transform a mobile device or platform into a medical device.”32Accordingly, the FDA proposes to exert its regulatory efforts toward mobile apps whose functionality could present a risk to patient safety if it were to not function as intended.33The functionality of a mobile app is subject to regulation if its intended use is for the diagnosis, cure, mitigation, treatment, or prevention of a disease, or if it has an effect on bodily functions.34However, while the majority of mobile apps do not function as a medical device, the guidance lacks clear regulation over the key component of clinical decision support, which can provide real-time data about patients to enhance healthcare and decision making in a clinical workflow.35
mHealth Opportunities in Clinical Research
Patient Recruitment
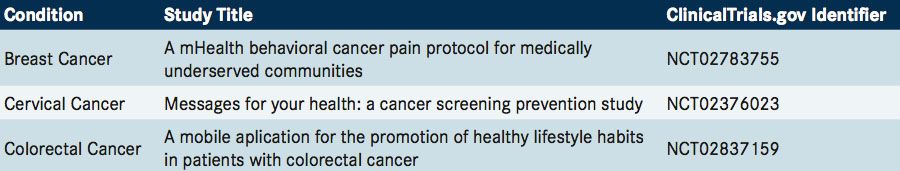
Traditional methods of patient recruitment remain one of the biggest and costliest bottlenecks in clinical research. With a trend in clinical research toward targeted patient populations, conventional methods of recruitment can be insufficient as many clinical trials fail to meet recruitment goals.36In contrast, Mount Sinai’s Asthma ResearchKit app registered nearly 50,000 users, of which 7593 patients were then enrolled in a 6-month research study (recruitment, consent, and enrollment were conducted entirely via a smartphone) that evaluated asthma treatment, geolocation, air quality, and patient-reported data. The study included a prospective collection of longitudinal and multidimensional data to analyze reports of asthma symptoms in regions affected by heat, pollen, or wildfires, as well as research challenges associated with the use of mHealth.37Like much internet-based research, limitations included selection bias, identity uncertainty, and high attrition rates.37Apps such as Clinical Trial Seek, from Noratis, help patients and researchers search for cancer trial information based on the National Institutes of Health’s database (clinicaltrials.gov).
Once patients are enrolled, however, barriers exist that can affect retention. A digital health study evaluating factors associated with dropout during recruitment of an mHealth-based randomized controlled trial reported lack of following interest in the trial, motivation to use mobile technology-based interventions, and lack of capacity (patients expressed inability to use mobile phones or text messaging) as factors affecting the attrition rate.38In addition, participant demographic characteristics (age, gender, education, and employment) were seen as dropout predictors. Whereas perceived stigma and patient illness have been identified in previous studies as barriers to recruitment, age and gender were the major factors in uencing recruitment and refusal.38Accordingly, an important step to recruiting patients with mHealth is to identify the feasibility within different patient groups and recognize the various factors that will help facilitate or inhibit patient engagement.
Collection of Reliable Health Data
There is considerable opportunity for clinicians to receive urgent information and act outside of the hospital setting. However, the timeliness and resources needed for the data entry of source documents continue to be a major challenge for healthcare as it is used to create patient history, fulfill regulatory requirements, and enable clinician decisions. Data collection is facilitated by mHealth technology that is designed to take place in real time and can be more comprehensive and efficient compared with established methodologies. The availability of more frequently measured data can support research by academic medical centers or pharmaceutical companies to further develop the safety profile and pharmacology of drugs, as well as identify drug interactions, to better evaluate the risk/benefit assessment. This approach allows for increased clinical observations for each patient to optimize data and improve outcomes.5Overall, mHealth technology combined with data collection enable the opportunity to capture a more comprehensive view of a patient’s health.
mHealth also could provide an opportunity for a new type of communication. The traditional periodic patient visit has the potential to be combined with more frequent, continuous digital communication for more effective care. This increased communication has the potential to complement the classic approach and encourage patients to better understand their health and become active participants in their care. A fully implemented mHealth system may result in fewer physician visits, better symptom management, and decreased healthcare. Study results have shown time-intensive tasks being replaced through mHealth technology, although more evidence is needed.39,40
Patient Engagement
A key element of mHealth technology is the ability to promote patient engagement with the healthcare system and its providers, which may lead to better clinical outcomes. Patients have more opportunities to report and log adverse effects (AEs) from medications, receive notifications and reminders to promote adherence, follow up on appointments, and manage disease. Furthermore, clinicians who have access to this type of data can develop a better understanding of a patient’s overall health.
There is a growing body of evidence that even simple interventions, such as text message reminders, improves adherence in a variety of chronic diseases.41Prospective research remains limited, but early studies indicate that digital mHealth interventions can improve patient engagement and adherence to treatment.42,43In a systematic evaluation of randomized controlled trials for behavioral change, reports of smoking cessation occurred after 1 week, as pregnant smokers who received mobile phone-based counseling intervention and text messages were shown to be more likely to set a quit date.44-46Moreover, the results of trials evaluating disease management showed improved cure rates and reported benefits in cardiopulmonary resuscitation processes with smartphone based instruction and feedback application for tuberculosis in those receiving medication reminders.47,48
Treatment adherence is a critical issue for oncology as studies have consistently shown that non-adherence leads to worse outcomes, including decreased survival.49Adherence to oral chemotherapy has been shown in empirical studies to range from 50% to 89%, depending on the definition of adherence and the study methodology.50-52In a randomized phase III study, a Web-mediated follow-up application (Moovcare), improved overall survival and quality of life in patients with stage III/IV lung cancer.53Patients assigned to the Web-application conducted a weekly self-assessment of their symptoms.; 12 symptoms were analyzed and reported to the oncologist. Triggered e-mail alerts (based on an algorithm assessing specific changes in symptoms) were sent to the physician to confirm the need for anticipated visits or supportive care options. The median survival of patients utilizing the application was 19 months compared with 12 months for those who received standard follow-up care. Overall quality of life, assessed using evaluation tests, including Functional Assessment of Cancer Therapy-Lung, Functional Assessment of Cancer Therapy-General, and Trial Outcome Index was also improved, and there was a 50% decrease in the average number of imaging tests per patient per year in the Web-application group. The Web-based application provided a continuous feedback mechanism for patients between visits to their oncologist, which resulted in early detection of complications and signs of relapse for earlier care. Thus, approaches that included monitoring of emerging toxicity prompting the healthcare team to conduct proactive symptom management would be expected to provide value in improving medication adherence in oncology. Another randomized controlled trial demonstrated higher compliance with using internet follow-up for patients with esophageal cancer.54Overall, ther results of studies using mHealth technology have shown higher compliance, earlier detection of relapse, increased access to health in remote areas, enhancement in the development of healthcare professionals, and lower travel costs.55-58
Evaluation of Toxicities of Novel Therapeutics
Novel therapeutics such as targeted therapies and immunotherapy have improved clinical outcomes in oncology but have different toxicity profiles than standard treatments (eg, chemotherapy, radiation). By allowing patients to evaluate their adverse effects on a continual basis, mHealth provides the opportunity to identify and manage toxicities earlier. Throughout all phases of oncology drug development, the reporting of symptomatic AEs is a crucial component of understanding the efficacy and quality of potential treatments and their impact on patients. The current method of collecting AEs in oncology clinical trials is based on physician reporting using the Common Terminology Criteria for Adverse Events. Clinicians report AEs from oncology treatment on a scale of can range from 0 to 5, each number representing severity levels of none, mild, moderate, severe, life-threatening, or death, respectively.59However, in an analysis comparing the reporting of symptomatic toxicity by patients and physicians in 3 randomized trials during cancer treatment, under-reporting by physicians ranged from 40% to 74.4%.60Thus, electronic patient-reported measures of AEs in oncology clinical trials can be used in early phase studies to generate initial information about AEs and to support selection of tolerable dose levels and schedules, measure baseline symptoms, and characterize symptomatic AE profiles.
In comparative trials, patient-reported AEs be used to differentiate tolerability between treatments, and in phase IV postmarketing studies, patient-reported AEs can be used to evaluate long-term safety.61As patients seek guidance on how to integrate their symptoms/knowledge into understanding their health condition, providers can develop shared goals based on patient-reported experiences to help explain symptoms and other findings to manage care in guided discovery, which emphasizes empiricism and use of the scientific method.62
Regulatory and Legal Perspective on mHealth Research
The FDA’s Use of Electronic Informed Consent in Clinical Investigations provides recommendations for institutional review boards (IRBs), but lacks explicit direction, leaving the boards to establish their own policies.63This creates variability in how IRBs review mHealth research and its potential risks to participants. An IRB review of mHealth research must address challenges such as the lack of face-to-face interaction, minor consent, responsibility of data integrity, privacy, security, confidentiality, and the collection of unrelated study data (eg, geographical location, personal contacts, Internet searches).64
Novel Research Methods to Assess mHealth Applications
With more than 160,000 mHealth apps available, there is much concern about validating the clinical safety and quality of mHealth technology.65Research has shown ongoing interest in developing methods to evaluate medical apps so physicians and consumers can be advised on their proper use. Currently, systematic reviews and novel methods are being investigated to assess medical apps for clinical safety. For example, Rx-Universe (from Mount Sinai) enables physicians to log into a patient’s EHR and digitally prescribe evidence-based mobile health applications, educational material, or patient satisfaction surveys.
Conclusion
As patients advocate for their place in the healthcare system, mHealth provides opportunities and resources for patients and providers to collaborate on healthcare and promote shared decision making for improved health and health outcomes. However, adoption of this technology requires that patients, providers, and informatics systems align despite the inherent challenges in mHealth adoption, EHR integration, or privacy, security, and condidentiality. As a disruptive technology, mHealth offers an opportunity to improve on the quality and safety of healthcare.
Acknowledgements: We acknowledge the support from NRG Oncology and Prostate Cancer Foundation.
References:
- Badawy S, Thompson A,Liem, R. Health-related smartphone apps: status update for hem-onc practitioners. The Hematologist. 2015;12(4). doi: 10.4161/hem.
- HIMSS Analytics. 3rd annual HIMSS Analytics mobile survey. HIMSS website. http://www.himss.org/3rd-annual-himss-analytics-mobile-survey-full- results?ItemNumber=41967. Published February 23, 2014. Accessed May 1, 2017.
- Kay M, Santos J, Takane, M. mHealth: new horizons for health through mobile technologies. World Health Organization website. http://www.who.int/goe/ publications/goe_mhealth_web.pdf. Published 2011. Accessed May 1, 2017.
- Moumtzoglou A. M-health Innovations for patient-centered care. IGI Global. 2016 doi: 10.4018/978-1-4666-9861-1.ch016.
- Patel S, Hemmige V, Street Jr RL, Viswanath K, Arya M. Activating racial and ethnic minorities to engage in preventive health: patient preferences for health reminders. J Participat Med. 2017;9:e8.
- Wood WA. Bennett AV, Basch E. Emerging uses of patient generated health data in clinical research. Mol Oncol. 2015;9(5):1018-1024. doi: 10.1016/j. molonc.2014.08.006.
- Ventola CL. Mobile devices and apps for health care professionals: uses and bene ts. P T. 2014;39(5):356-364..
- Chung AE, Skinner AC, Hasty SE, Perrin EM. Tweeting to health: a novel mHealth intervention using Fitbits and Twitter to foster healthy lifestyles [ePub ahead of print]. Clin Pediatr (Phila). 2016. pii: 0009922816653385.
- Kessel KA, Vogel MM, Schmidt-Graf F, Combs SE. Mobile apps in oncology: a survey on health care professionals’ attitude toward telemedicine, mHealth, and oncological apps. J Med Internet Res. 2016;18(11):e312.
- Boulos MN, Brewer AC, Karimkhani C, Buller DB, Dellavalle RP. Mobile medical and health apps: state of the art, concerns, regulatory control and certi cation. Online J Public Health Inform. 2014;5(3):229. doi: 10.5210/ojphi.v5i3.4814.
- mHealth: history, analysis, and implementation. In: Waegemann CP, ed. M-Health Innovations for Patient-Centered Care. IGI Global;2016:1-19. http://www.igi- global.com/chapter/mhealth/145002.
- McGillicuddy JW, Weiland AK, Frenzel RM, et al. Patient attitudes toward mobile phone-based health monitoring: questionnaire study among kidney transplant recipients. J Med Internet Res. 2013;15(1):e6. doi: 10.2196/jmir.2284.
- Cox A, Lucas G, Marcu A, et al. Cancer survivors’ experience with telehealth: a systematic review and thematic synthesis. J Med Internet Res. 2017;19(1):e11. doi: 10.2196/jmir.6575.
- Viers BR, Lightner DJ, Rivera ME, et al. E ciency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68(4):729-735. doi: 10.1016/j.eururo.2015.04.002.
- Nelson R, Joos I, Wolf DM. Social media for nurses: educating practitioners and patients in a networked world. Springer Publishing Company. ISBN 13 9780826195883; 2012.
- Sclafani J, Tirrell TF, Franko OI. Mobile tablet use among academic physicians and trainees. J Med Syst. 2013;37(1):9903. doi: 10.1007/s10916-012-9903-6.
- Yamin CK, Emani S, Williams DH, et al. The digital divide in adoption and use of a personal health record. Arch Intern Med. 2011;171(6):568-574. doi: 10.1001/ archinternmed.2011.34.
- The National Academy of Sciences, Engineering, and Medicine. The role of telehealth in an evolving health care environment-workshop summary. National Academies of Sciences website. http://www.nationalacademies.org/hmd/Reports/2012/The-Role-of-Telehealth-in-an-Evolving-Health-Care-Environment. aspx. Published November 20, 2012. Accessed May 1, 2017.
- Wood J, Mulrennan S, Hill K, Cecins N, Morey S, Jenkins S. Telehealth clinics increase access to care for adults with cystic brosis living in rural and remote Western Australia. J Telemed Telecare [ePub ahead of print]. 2016. pii: 1357633X16660646
- Hennemann-Krause L, Lopes AJ, Araújo JA, Petersen EM, Nunes RA. The assessment of telemedicine to support outpatient palliative care in advanced cancer. Palliat Support Care. 2015;13(4):1025-1030. doi: 10.1017/ S147895151400100X.
- Sabesan S. Medical models of teleoncology: current status and future directions. Asia Pac J Clin Oncol. 2014;10(3):200-204. doi: 10.1111/ajco.12225.
- Washington PK, Tews H, Nguyen DT, et al. Use of telemedicine in the delivery of survivorship care plans among breast cancer survivors: lessons learned at Kaiser Permanente East Bay. J Clin Oncol. 2017;35(suppl 5S; abstr 77).
- Cole-Vadjic, KK, Crews JR. (2016). Use of telemedicine to expand access to survivorship care. Cancer Survivorship Symposium: Advancing Care and Research A Primary Care and Oncology Symposium. San Francisco, CA; January 15, 2016. https://www.mdlinx.com/internal-medicine/conference-abstract.cfm /56132/?nonus=0&searchstring=&coverage_day=0&page=1. Accessed May 1, 2017.
- Galsky MD, Shahin M, Olson A, et al. Telemedicine-enabled clinical trial of metformin in patients (pts) with biochemically-recurrent prostate cancer (PCa). J Clin Oncol. 2017;35:(suppl 6s; abstr 243).
- Velis SA, Dreyer NJ, Andersen N, et al. Virtual consults: a paradigm shift for second opinions in oncology care. J Clin Oncol. 2016;34(suppl 15S; abstr e18049).
- Nelson R, Staggers N. Health informatics: An interprofessional approach. Elsevier Health Sciences. ISBN-10: 0323100953. 2017.
- Mandel JC, Kreda DA, Mand KD, Kohane IS, Ramoni R. SMART on FHIR: a standards-based, interoperable apps platform. J Am Med Inform Assoc. 2016;23(5):899-908. doi: 10.1093/jamia/ocv189.
- Bloom eld RA Jr, Polo-Wood F, Mandel JC, Mand KD. Opening the Duke electronic health record to apps: Implementing SMART on FHIR. Int J Med Inform. 2017;99:1- 10. doi: 10.1016/j.ijmedinf.2016.12.005.
- Yu P, Wu MX, Yu H, Xiao GQ. The challenges for the adoption of m-health. University of Wollongong Australia website. http://ro.uow.edu.au/cgi/viewcontent.cgi?article =1495&context=infopapers. Published 2006. Accessed May 1, 2017
- Gagnon MP, Ngangue P, Payne-Gagnon J, Desmartis M. m-Health adoption by healthcare professionals: a systematic review. J Am Med Inform Assoc. 2016;23(1):212-220. doi: 10.1093/jamia/ocv052.
- Seto E, Leonard KJ, Cafazzo JA, Barnsley J, Masino C, Ross HJ. Perceptions and experiences of heart failure patients and clinicians on the use of mobile phone- based telemonitoring. J Med Internet Res. 2012;14(1):e25. doi: 10.2196/jmir.1912.
- FDA. Mobile medical applications. Guidance for industry and Food and Drug Administration Sta . FDA website. https://www.fda.gov/downloads/MedicalDevices/.../ UCM263366.pdf. Published February 9, 2015. Accessed May 1, 2017.
- Cortez NG, Cohen IG, Kesselheim AS. FDA regulation of mobile health technologies. N Engl J Med. 2014;371(4):372-379. doi: 10.1056/NEJMhle1403384.
- Martínez-Pérez B, de la Torre-Díez I, López-Coronado M, Sainz-de-Abajo B, Robles M, García-Gómez JM. Mobile clinical decision support systems and applications: a literature and commercial review. J Med Syst. 2014;38(1):4. doi: 10.1007/s10916-013-0004-y.