Sipuleucel-T Shows Potential With New Trial Data, But Questions Regarding Clinical Relevance Remain
Since the approval of sipuleucel-T for the treatment of minimally symptomatic metastatic castration-resistant prostate cancer in 2010, barriers to administration and the approval of competing drugs has tampered wide adoption of its use.
Leonard G. Gomella, MD
Since the approval of sipuleucel-T (Provenge) for the treatment of minimally symptomatic metastatic castration-resistant prostate cancer in 2010, barriers to administration and the approval of competing drugs has tampered wide adoption of its use. Despite it being the first FDA-approved personalized cancer vaccine with positive efficacy and safety data, its use has not been widely adopted.
Although the trajectory of this groundbreaking therapy has been different from that of checkpoint blockade immunotherapy, advocates for sipuleucel-T point out that the public has become more aware of and interested in cancer vaccines now, and they believe the product has the potential to benefit a larger group of patients. Dendreon Pharmaceuticals, the manufacturer of sipuleucel-T, last year began a new effort to expand its market by launching ProVent (NCT03686683), a phase III randomized trial testing the effectiveness of sipuleucel-T versus active surveillance in decreasing histologic progression of low-grade prostate cancer (FIGURE).1
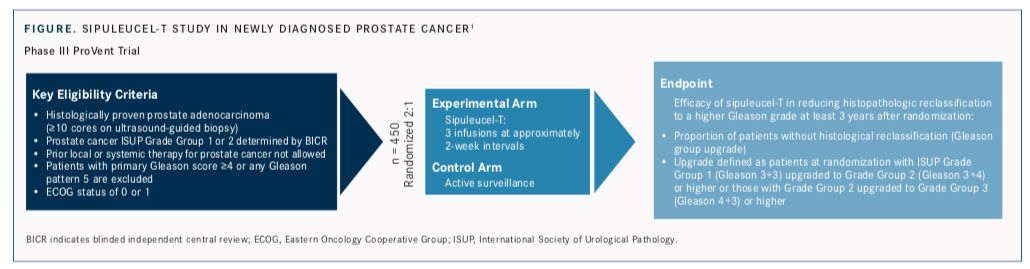
ProVent “has a lot of potential, depending on how quickly it can accrue. Obviously, that is a very, very big space. There’s lots of interest in patients with newly diagnosed, low-grade prostate cancer on active surveillance,” said Raoul S. Concepcion, MD, director of The Comprehensive Prostate Center and clinical associate professor at Vanderbilt University School of Medicine, both in Nashville, Tennessee. He has served as a physician adviser to Dendreon.
Several other trials are testing combinations or sequencing of sipuleucel-T with radiotherapy, checkpoint inhibitors, and other therapies. “It will maintain itself as a go-to medication for metastatic castration-resistant prostate cancer, but the future is in combination therapylearning how to mix it not only with chemotherapy, but with androgen-receptor pathway blockers or with other immunotherapeutic agents that might enhance the overall response to sipuleucel-T,” said Leonard G. Gomella, MD, director of the Kimmel Cancer Center Network at Thomas Jefferson University Hospital in Philadelphia.
Impact on PSA
Sipuleucel-T treatment begins with leukapheresis, typically at a physician’s office or blood-collection center. The harvested white blood cells, most importantly dendritic antigen-presenting cells, are sent to a manufacturing facility. They are incubated with a recombinant fusion protein consisting of prostatic acid phosphatase (PAP), an antigen that is highly expressed in most prostate cancer cells, and granulocyte-macrophage colony-stimulating factor.
Three or 4 days after the initial blood draw, the activated cells are reinfused into the patient, activating an immune system response against PAP-expressing prostate cancer cells. The procedure is conducted 3 times over a period of 6 weeks.2
Attitudes toward sipuleucel-T have been heavily shaped by the fact that the therapy has not demonstrated significant impacts on lowering prostate-specific antigen (PSA) levels and on slowing disease progression in radiographic imaging studies. The first phase III trial, D9901 (N = 127), did not achieve a statistically significant improvement in the primary endpoint of median time to progression; PSA progression was not included in the primary endpoint.3The study was not powered for overall survival (OS), but investigators were surprised to find the sipuleucel-T group had a median survival advantage of 4.5 months over the placebo group (25.9 vs 21.4 months; log-rank P = .01). The estimated survival rate at 36 months was 34% for sipuleucel-T and 11% for placebo (P = .005).
The data were pooled with findings from another phase III trial, D9902A, and the integrated results showed a similar median survival benefit of 4.3 months (HR, 1.50; 95% CI, 1.10- 2.05; log rank P = .011).4Yet the failure to meet the original endpoint and the lack of evidence of direct antitumor effect, along with other concerns, sowed skepticism among some members of the FDA advisory committee reviewing the data.
In 2007, the agency issued a complete response letter to Dendreon and asked for more data.5Gomella said immunotherapy was still new and the FDA wanted more robust evidence that it was effective. At the time, “immunotherapy in prostate cancer was heresy,” he said. “Whoever thought that prostate cancer would respond to an immune agent? There was a lot of hesitation.”
Dendreon followed up with the pivotal IMPACT trial, in which 512 patients were assigned in a 2:1 ratio to receive either sipuleucel-T (n = 341) or placebo (n = 171), with a primary endpoint of OS.6 In the sipuleucel-T group, there was a relative reduction of 22% in the risk of death as compared with the placebo group (HR, 0.78; 95% CI, 0.61-0.98;P= .03). This represented a 4.1-month improvement in median survival (25.8 vs 21.7 months), confirming the findings of the previous studies.
The 36-month survival probability in IMPACT was 31.7% in the sipuleucel-T group versus 23.0% in the placebo group. The median time to objective disease progression did not differ significantly between the study groups (14.6 vs 14.4 weeks, respectively).
Among patients with PSA assessments after baseline, reductions of at least 50% on 2 visits at least 4 weeks apart were observed in 8 of 311 patients (2.6%) in the sipuleucel-T group, compared with 2 of 153 patients (1.3%) in the placebo group. Adverse events (AEs), all-grade and grades ≥3, were comparable between the 2 treatment groups; all-grade AEs more frequently reported in the sipuleucel-T patients included chills, fever, and headache.
Marketplace Challenges
The decision to approve sipuleucel-T was hailed by patients and advocacy groups, but the new therapy did not fare well in the marketplace, earning Dendreon a little more than half of the expected product revenues in 2011. It did not become available in other countries. Physicians at some US medical centers declined to use sipuleucel-T because of doubts that it worked, while fearing angry responses from patients who demanded it.7
At the same time, other patients who would benefit from sipuleucel-T could not always be convinced to take the therapy. “The hard part was, we didn’t know if it worked in patients or not because there was no objective decrease in PSA and there was no objective decrease in lymph nodes. Bone scans are always tough to evaluate in patients. So there was nothing objective from a patient [or] a provider point of view to tell us if this drug worked or not,” said Sandy Srinivas, MD, a professor of medicine (oncology) at Stanford University Medical Center and member of the National Comprehensive Cancer Network guidelines panel for prostate cancer.
It is possible that sipuleucel-T would eventually have an observable effect on tumor progression, but trial investigators may have felt compelled to switch therapies before that point because their patients’ PSA levels were rising, Gomella said. “The patient gets nervous, the doctor gets nervous, and they might not have given the drug enough of a chance to really have an impact before they moved to the next treatment,” he said.
Uncertainty about the therapy’s mechanism stoked an unusual controversy. In 2012, a paper in the Journal of the National Cancer Insti- tute hypothesized that sipuleucel-T appeared to extend survival only because older men (aged ≥65 years) in the control group had worse OS outcomes versus younger participants, due perhaps to differences in the harvested cells used in the placebo group (two-thirds of cells were frozen and not reinfused).8Dendreon and the IMPACT investigators rejected the claims.
Another obstacle to adoption has been what Concepcion called the “cost density” of sipuleucel-T, which runs about $100,000. That adminis- tration is also complex, which put some doctors off the therapy, said Gurkamal Chatta, MD, clinical chief of genitourinary medicine at Roswell Park Comprehensive Cancer Center in Buffalo, New York.
“There are multiple layers to coordinating everything. You have to arrange for the leukapheresis, then you have to get the patient to come in to receive the product. It’s really 6 trips rather than 3 trips,” he said.
Optimizing Patient Selection
Sipuleucel-T’s surprising OS benefit was both the key to its eventual approval and a complicating factor in the trial designs. One of the “major sell- ing points” for trial patients was the availability of sipuleucel-T as a salvage therapy for those in the control arm, Gomella said. When those patients’ cancer progressed, they had the option of having their cryopreserved blood cells activated with the fusion protein and reinfused, extending their survival but muddying the trial results.
“The IMPACT trial was its own worst enemy, in a way, and actually probably underrepresented the survival advantage from sipuleucel-T,” said Gomella, who coauthored an analysis of the salvage therapy. “This was not the cleanest trial in the world, because the placebo arm was not a pure placebo arm. If you started to look at the net benefit, if you took the sipuleucel-T salvage arm out, you actually saw much, much better survival in the treatment arm.”
About two-thirds (165 of 249) of the control patients received the frozen product, APC8015F. After adjusting for independent predictors of postprogression survival, a statistical analysis showed them trending toward improved survival (HR, 0.78; 95% CI, 0.54-1.11; P = .17).9 Estimated median OS benefit for sipuleucel-T versus control adjusted for APC8015F treatment was 8.1 months, if APC8015F was equally effective as sipuleucel-T.
“Theoretically, it was a doubling of the survival, which is pretty impressive,” Gomella said.
Another posthoc analysis of the IMPACT data showed greater survival benefit in patients who had lower PSA when they received sipuleucel-T.10When the trial results were subdivided by baseline PSA into quartiles, patients in the lowest baseline PSA quartile (≤22.1 ng/mL) showed an estimated median survival time of 41.3 months, versus 18.4 months in the highest quartile (>134.1 ng/mL). In the control arm, the estimated median survival times were 28.3 months versus 15.6 months for patients in the lowest versus highest baseline PSA quartile.
“You need to treat people who have maybe intermediate- to low-risk disease, where the PSA is not taking off but is gradually going up, where essentially you give the immune system a chance to ‘harness’ the cancer,” Chatta said. “If somebody doesn’t have super-aggressive disease...you can buy some time before you commit them to essentially lifelong therapy on a daily basis either with abiraterone [Zytiga] or enzalutamide [Xtandi].” The other therapies available for these patients are docetaxel and radium-223 (Xofigo).
Further data have come from PROCEED, a phase IV registry study that Dendreon set up. A signif- icant finding was the greater benefit for African American men with mCRPC. A multivariate analysis matching 420 Caucasian patients with 210 African American patients by baseline PSA found OS was 39.5 months for the latter group versus 28.1 months for the former (P <.001; HR, 0.665; 95% CI, 0.530-0.835).11Younger age, lower PSA or alkaline phosphatase, and higher hemoglobin levels were independently associated with longer OS, as was no prior chemotherapy
Ongoing Studies
The ProVent trial that launched last year takes a different tack, using standard needle biopsies to measure histopathologic disease progression in men on active surveillance. The phase III trial will be conducted at approximately 50 sites across the United States with a targeted enrollment of 450 participants. They are being randomized 2:1 to receive sipuleucel-T or placebo.
Immunotherapy combinations are also beginning to get attention. In a recent, small phase I trial, 9 men with mCRPC received sipuleucel-T followed by escalating doses of ipilimumab (Yervoy).12The combination was well tolerated. Statistically significant increases in serum immunoglobulin G (IgG) and IgG-IgM levels specific for PAP and PA2024 were observed after administration of sipuleucel-T, and levels increased further with use of ipilimumab. A phase II trial combining sipuleucel-T with immediate versus delayed ipilimumab remains active (NCT01804465).
Other current trials combine sipuleucel-T with atezolizumab (Tecentriq) in patients with asymptomatic or minimally symptomatic mCRPC (NCT03024216) and with stereotactic ablative body radiation (NCT01818986).
References:
- Fitzhugh M. Dendreon seeks expanded role for Provenge in new early stage prostate cancer trial. Dendreon website [reprinted from BioWorld. 2018;29(98)]. dendreon.com/Portals/12/dendreon-pharmaceuticals-bioworld-reprint.pdf. Accessed January 8, 2019.
- Questions and answersProvenge. US FDA website. www.fda.gov/biologicsbloodvaccines/cellulargenetherapyproducts/approvedproducts/ucm210037.htm. Updated March 16, 2018. Accessed January 25, 2019.
- Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089-3094. doi: 10.1200/JCO.2005.04.5252.
- Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670-3679. doi: 10.1002/cncr.24429.
- Dendreon receives complete response letter from FDA for Provenge BLA [news release]. Seattle, WA: Dendreon Corporation; May 9, 2007. fiercebiotech.com/biotech/press-release-dendreon-receives-complete-response-letter-from-fda-for-provenge-bla. Accessed January 25, 2019.
- Kantoff PW, Higano CS, Shore ND, et al; IMPACT Study Investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422. doi: 10.1056/NEJMoa1001294.
- Begley S. Insight: new doubts about prostate-cancer vaccine Provenge. Reuters website. reuters.com/article/us-provenge/insight-new-doubts-about-prostate-cancer-vaccine-provenge-idUSBRE82T07420120330. Published March 30, 2012. Accessed January 8, 2019.
- Huber ML, Haynes L, Parker C, Iversen P. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104(4):273-279. doi: 10.1093/jnci/djr514.
- George DJ, Nabhan C, DeVries T, et al. Survival outcomes of sipuleucel-T phase III studies: impact of control-arm cross-over to salvage immunotherapy. Cancer Immunol Res. 2015;3(9):1063-1069. doi: 10.1158/2326-6066.CIR-15-0006.
- Schellhammer PF, Chodak G, Whitmore JB, et al. Lower baseline prostate-specific antigen is associated with a great overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology. 2013;81(6):1297-1302. doi: 10.1016/j.urology.2013.01.061.
- Sartor AO, Armstrong A, Ahaghotu C, et al. PD24-12 Overall survival analysis of African American and Caucasian patients receiving sipuleucel-T: preliminary data from the PROCEED registry. J Urol. 2017;197(4S):e456-e457. doi: 10.1016/j.juro.2017.02.1089.
- Scholz M, Yep S, Chancey M, et al. Phase I clinical trial of sipuleucel-T combined with escalating doses of ipilimumab in progressive metastatic castrate-resistant prostate cancer. Immunotargets Ther. 2017;6:11-16. doi: 10.2147/ITT.S122497.
