Existing Drugs May Offer Treatment Opportunities in Pancreatic Ductal Adenocarcinoma
Findings from a study of tumor samples collected from patients with pancreatic ductal adenocarcinoma revealed that 17% of tumors harbored a known genomic alteration for which targeted therapies have already been developed, conferring feasibility to implementing precision medicine in a disease that has seen little progress in the development of effective therapeutic agents.
Findings from a study of tumor samples collected from patients with pancreatic ductal adenocarcinoma (PDAC) revealed that 17% of tumors harbored a known genomic alteration for which targeted therapies have already been developed, conferring feasibility to implementing precision medicine in a disease that has seen little progress in the development of effective therapeutic agents.1
Genomic mutations in KRAS, TP53, CDKN2A, and SMAD4 are most frequently observed in PDAC, yet no currently available targeted agents are directed at these alterations. Adding to this is the heterogeneous characterization of PDAC because of numerous mutations present at low levels (<5%), contributing to the slow evolution of systemic therapies.
“The complex genomic heterogeneity in otherwise histologically similar PDACs suggests a one-size-fits-all approach to treating patients will not be successful,” said Aatur D. Singhi, MD, PhD, and colleagues in their report published in Gastroenterology. “Targeted genomic profiling of known genomic alterations across multiple tumor types highlights potentially targetable and predictive biomarkers for treatment in a significant subset of PDACs.”
Targeted genomic profile analysis was performed using 3594 PDAC samples from an international cohort of patients. In addition, investigators assessed for tumor mutation burden (TMB) in 1021 samples (28%) and microsatellite instability (MSI) status in 2563 (71%).
Investigators categorized genomic alterations based on signaling pathways using published literature as follows: receptor tyrosine kinase/ Ras/mitogen-activated protein kinase (RTK/Ras/ MAPK) activation, DNA damage repair, cell cycle regulatory, transforming growth factor (TGF)β signaling, histone modification, switching/sucrose nonfermenting (SWI/SNF) complex, PI3K/mTOR signaling, Wnt/β-catenin pathway, Notch signaling, angiogenesis, and Hedgehog signaling (FIGURE).
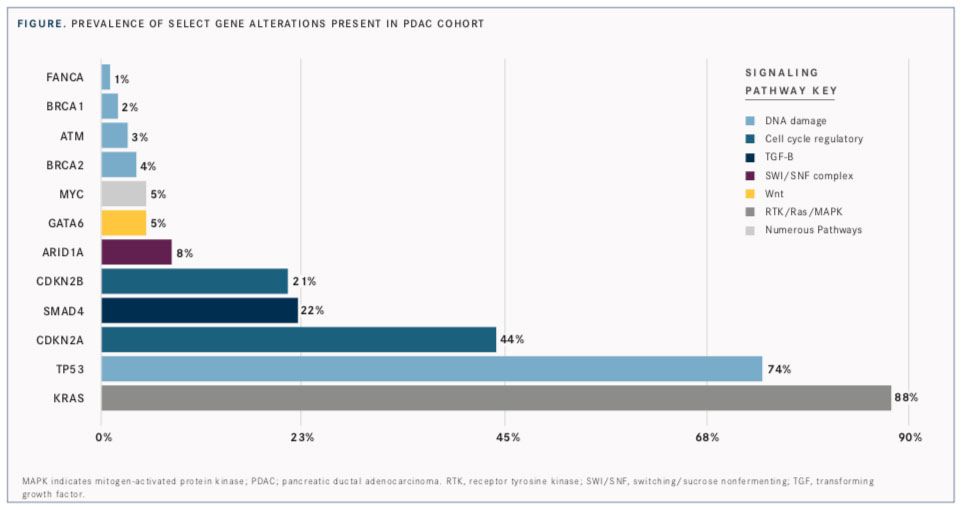
Alterations were further divided into clinically relevant genomic alterations for which there are available anticancer drugs on the market or in clinical trials. Those identified in the PDAC samples (17%) were classified by Singhi et al as either targetable mutations in RTK/Ras/MAPK signaling or predictive biomarkers for treatment in the DNA damage repair pathways.
RTK/Ras/MAPK Activation
In total, 19,120 genomic alterations were identified in 317 genes, with KRAS alterations occurring with the highest frequency. Contrary to established data, about 12% of samples wereKRASwild-type; previous studies showed an absence of KRAS mutations in <5% of PDACs. Investigators hypothesized that the discordance may be due to differences in the tumor samples collected. Prior reports often used specimens from pancreatic surgical resections, whereas the clinical cohort in the study included both resectable and unresectable disease samples.
The identification of a significant number ofKRASwild-type PDACs creates the opportunity to detect alternative drivers of targeted therapy within the RTK/Ras/MAPK pathway. Kinase fusion genes, such as ALK, BRAF, RET, and NTRK1, were the most commonly reported puta- tive driver alterations in this group.
Limited data regarding the use of available targeted therapies in PDAC exist; however, a presentation from the 2018 Gastrointestinal Cancers Symposium showed that the investigational pan-ALK/TRK/ROS1 inhibitor entrectinib produced partial responses in all 3 patents, 2 withNTRKgene-fusion and 1 withROS1fusionpositive 2 pancreatic cancer. Other mutations include BRAF deletions, which may be targetable with the MEK1/2 inhibitor trametinib (Mekinist); ALK fusions, for which 1 of the FDA-approved ALK inhibitors may show activity; and other amplifications and missense mutations in genes involved in the RTK/Ras/MAPK pathway.
DNA Damage Repair
Although individual mutations in genes involved in the DNA damage repair pathway typically presented at frequencies <5%, they collectively affected 81% of PDACs in the cohort. The investigators said that germline alterations with the greatest implication for treatment are those present in the BRCA-FANC family (14%) and may serve as predictive biomarkers for sensitivity to therapies such as PARP inhibitors and platinum-based cytotoxic regimens.
No clinical trials are comparing the efficacy of platinum-based chemotherapy against other agents in patients with PDAC, but several ret- rospective analyses suggest that patients withBRCA-mutated disease see benefit in overall survival (OS) rates with these chemotherapy options compared with nonplatinum-based chemotherapy.
Early clinical trials of PARP inhibitors inBRCA-mutated PDAC demonstrated positive responses. In a phase II trial evaluating olaparib (Lynparza) in patients with different tumor types who harbored a germlineBRCA1/2 mutation, the response rate in 23 patients with pancreatic cancer who had received an average of 2 prior lines of chemotherapy for metastatic disease was 22% (95% CI, 7.5%-43.7%).3Of those patients, there was 1 complete response and 4 partial responses; 8 patients had stable disease and 40.9% were alive at 12 months.
Promising data from olaparib trials led to the phase III randomized POLO trial (NCT02184195) of maintenance olaparib monotherapy versus placebo in patients with germlineBRCA-mutated metastatic pancreatic cancer whose disease has not progressed on frontline platinum-based chemotherapy. In October 2018, the FDA granted the PARP inhibitor orphan drug designation in the treatment of pancreatic cancer.4Then, in February 2019, Myriad Genetics announced that, based on the trial results, it would submit a supplementary premarket approval application to authorize its companion diagnostic test, BRCAnalysis CDx, for use in patients with pancreatic cancer.5 The test was able to successfully identify patients with metastatic pancreatic cancer who had BRCA mutations and would likely benefit from treatment with olaparib.
Issues With TMB and MSI-High Tumors
Immunotherapy has shown benefit for patients with MSI-high and TMB-high tumors; however, just 0.1% and 0.5% of PDACs in the cohort were assessed to be MSI high and TMB high, respectively.
MSI-high PDACs treated with the antiPD-1 inhibitor pembrolizumab (Keytruda), which was approved for a tumor-agnostic indication in mis-match-repair deficiency, had an overall response rate of 62% and a disease control rate of 75% in 8 patients in a phase II trial. Disappointingly, the prevalence of MSI-high status in patients with PDAC has been historically infrequent.
Uncertainty remains about the clinical relevance of TMB across tumors types. This is aggravated by the fact that individual disease types reflect different cutoff points for what is considered high and low expression of TMB.6In this study, investigators used ≥20 mutations per megabase of sequenced DNA as the cutoff for high TMB. Further analysis is needed to determine the upper limits of TMB expression in PDAC and what it means for use of immunotherapy in those tumors.
Applying Findings to Clinical Practice
Further correlations were observed between genomic alterations and presentation of disease. Primary PDACs were more commonly associated withKRASmutations, whereas metastatic disease was associated with CDKN2A, CDKN2B, MYC, MLL2, ERBB2, CDK6, RB1, and MYST3 alterations.
Subanalyses showed that patients with liver metastases more commonly harbored mutations, including TP53 and CDK6. Conversely, GNAS mutations were less likely to be present in the liver but were more often detected in lung metastases.
Patients ≥50 years were more likely to have genetic alterations in the KRAS, SMAD4, DNMT3A, and APC genes, whereas patients <50 more commonly hadBRCA1/2 alterations.
By gender, female patients were more likely to have alterations in KRAS, SMAD4, and CDKN2A compared with male patients, who often exhibited GNAS, KDM6A, ATM, and SF3B1.
The presence of GNAS alterations in PDAC was specific for tumors arising from intraductal papillary mucinous neoplasms (IPMNs) and has been shown to occur early in the progres- sion of these noninvasive precursors to PDAC. Therefore, genomic alterations in GNAS may be involved in the pathogenesis of IPMN-associated PDACs. These alterations can be assessed by aspirating pancreatic cyst fluid and may present an early detection screening method for this PDAC subtype.
References:
- Singhi AD, George B, Greenbowe JR, et al. Real-time targeted genome profile analysis of pancreatic ductal adenocarcinomas identifies genetic alterations that might be targeted with existing drugs or used as biomark- ers [published online March 2, 2019]. Gastroenterology. doi: 10.1053/j. gastro.2019.02.037.
- Pishvaian MJ, Rolfo CD, Liu SV, Multani PS, Maneval EC, Garrido-Laguna I. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. J Clin Oncol. 2018;36(suppl 4; abstr 521). doi: 10.1200/JCO.2018.36.4_suppl.521.
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244-250. doi: 10.1200.JCO.2014.56.2728. 4. Fourth orphan drug designation in the US for AstraZeneca and MSD’s Lynparza [news release]. Kenilwoth, NJ: AstraZeneca and Merck & Co, Inc; October 16, 2018. bit.ly/2pYa7c8. Accessed March 25, 2019.
- Myriad’s BRACAnalysis CDx Test successfully identified patients with metastatic pancreatic cancer who benefitted from treatment with olaparib [news release]. Salt Lake City, UT: Myriad Genetics, Inc; February 26, 2019. bit.ly/2V6MGeK. Accessed March 25, 2019.
- Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202-206. doi: 10.1038/s41588-018-0312-8.
