Sequential Mitomycin With BCG Is Shown to Be Safe in High-Risk NMIBC
Results from the phase 4 MITO-BCG study show safety of sequential mitomycin with BCG for the treatment of high-risk muscle invasive bladder cancer.
Treatment with added mitomycin chemotherapy and BCG, administered sequentially, in patients with high-risk nonmuscle-invasive bladder cancer (NMIBC) showed a safety profile similar to that of BCG alone. Results from the phase 4 MITO-BCG study (EUDRACT 2017-004540-37; NCT03790384) were presented during the 2021 Annual European Association of Urology (EAU) Congress.1
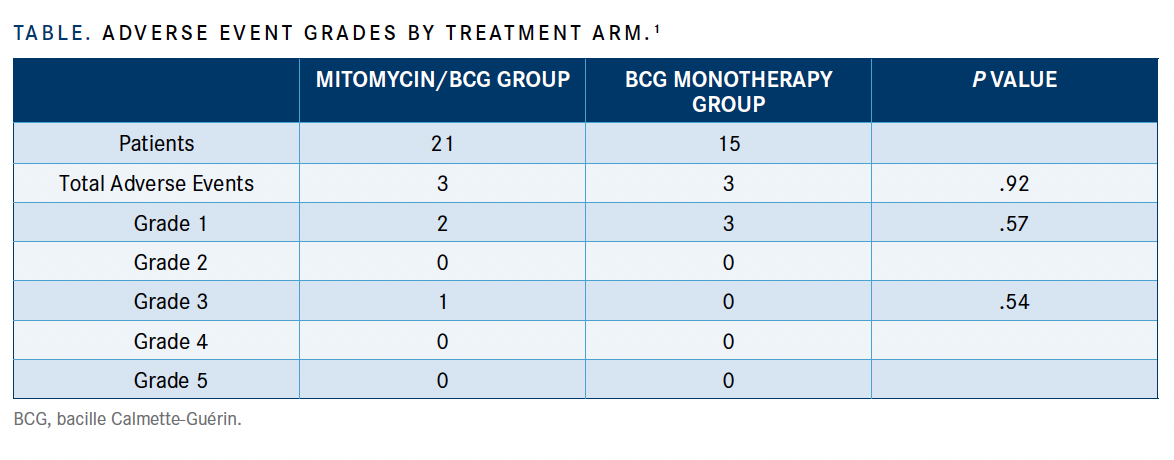
No significant differences in safety were reported between the 2 arms. Results showed that 6 patients experienced posttreatment adverse effects (AEs) overall, with 3 reported in the combination therapy arm. Five of the 6 AEs, which were grade 1 by Common Terminology Criteria for Adverse Events (TABLE), were urethral substenosis, fever, asthenia, fainting, and cystitis and did not require further treatment.
One grade 3 AE, acute epididymo-orchitis with abscess, was reported in the BCG-monotherapy arm and required orchiectomy. No grade 3 or higher AEs occurred in the combination arm.
“Preliminary results of our experience [in the MTIO-BCG study] support that sequential combination therapy with mitomycin and BCG is a [safe] treatment in high-risk NMIBC,” said study author Antonio Nacchia, MD, medical director of urology, IRCCS Oncology Reference Center in Italy, in a virtual presentation of the data during the meeting.
In the ongoing, open-label, phase 4 MITOBCG study, investigators are evaluating the efficacy and safety of sequential treatment with mitomycin and BCG combination therapy vs BCG alone in patients with high-risk NMIBC. The protocol had been approved by the Italian Medicines Agency in August 2018 and then by the Ethical Committee in Italy in December 2018; the protocol started in March 2019.
To be eligible for enrollment, patients must have high-risk NMIBC that was either: T1, grade 3, carcinoma in situ, multiple and recurrent large Ta, G1 or G2, or in the last EAU recurrence category of EAU/European Organisation for Research and Treatment of Cancer recurrence score greater than or equal to 10. Patients need to be between ages 40 and 75 years.
Those with low-risk NMIBC, muscle-invasive bladder cancer, or concomitant urothelial bladder cancer in the upper urinary tract, or those who had prior bladder or prostate surgery, prior prostate or bladder radiotherapy, urinary tract infection, chronic urinary retention or indwelling catheters, have neurologic issues, had prior BCG infections, prior or current chemotherapy for other malignancies, or residual tumor on re–transurethral resection of the bladder were excluded from enrollment. Additionally, patients could have a World Health Organization performance status between 3 and 4.
Thirty-six patients were randomized to receive standard BCG induction therapy as an instillation once weekly for 6 weeks with an 81-mg Connaught strain of BCG (n = 21) or the BCG protocol with 40 mg of mitomycin instillation given the day before BCG starts at once weekly for 6 weeks (n = 15). Cystoscopy and urine cytology were both conducted every 3 months along with annual CT scans. The median age of patients was 67 years.
The primary end point of the trial is recurrence rate, with overall toxicity being a secondary end point. Assessment of health-related quality of life serves as an exploratory end point. Trial enrollment will continue until 200 patients are included in the study, Nacchia concluded.
In April 2020, the FDA approved mitomycin gel (UGN-101; Jelmyto) as the first therapy to treat patients with low-grade upper tract urothelial cancer.2 Mitomycin gel uses the RTGel technology platform and is designed to permit longer exposure of mitomycin to urinary tract tissue, which allows for the nonsurgical treatment of these tumors. The therapy is administered to patients using standard ureteral catheters.
References:
1. De Nunzio C, Nacchia A, Lombardo R, et al. Safety evaluation of sequential Mitomycin and Bacillus Calmette-Guérin treatment versus Bacillus Calmette-Guérin monotherapy in patients with high risk non-muscle invasive bladder cancer: MITOBCG study (EUDRACT Number 2017-004540-37) preliminary results. Presented at: 36th Annual European Association of Urology Congress; July 8-12, 2021; virtual. Abstract P0447.
2. FDA approves first therapy for treatment of low-grade upper tract urothelial cancer. News release. FDA. April 15, 2020. Accessed August 20, 2021. https://bit.ly/2wGE4Es

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More