Safety Profile for Darolutamide Favorable in Nonmetastatic CRPC Treatment
A safety analysis from the phase 3 ARAMIS trial showed that tolerability with darolutamide was comparable with that of placebo in patients with nonmetastatic castration-resistant prostate cancer.
Karim Fizazi, MD, PhD

A safety analysis from the phase 3 ARAMIS trial (NCT02200614) showed that tolerability with darolutamide (Nubeqa) was comparable with that of placebo in patients with nonmetastatic castration-resistant prostate cancer (CRPC). Results from the analysis presented during the 2021 European Association of Urology (EAU) Congress showed a similar low cumulative incidence of hormone treatment–related adverse events (HTR AEs) with darolutamide compared with placebo.1
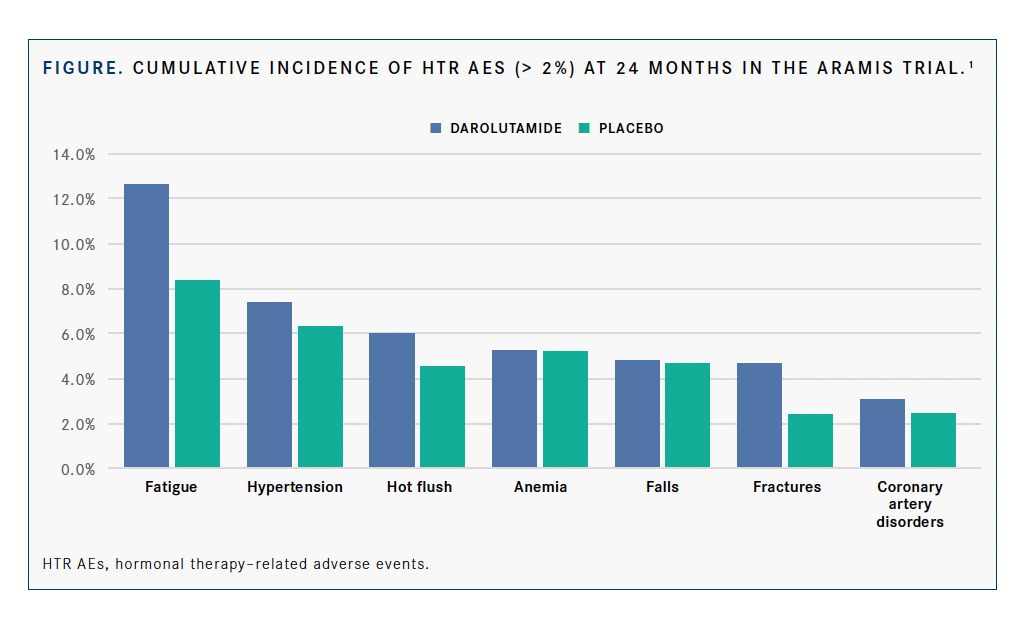
The cumulative incidences of HTR AEs that occurred in more than 2% of patients at 2 years during the double-blind treatment period in the darolutamide/androgen deprivation therapy (ADT) and placebo/ADT arms, respectively, included fatigue (12.6% vs 8.3%), hypertension (7.3% vs 6.3%), hot flush (6.0% vs 4.5%), anemia (5.8% vs 5.1%), falls (4.8% vs 4.7%), fractures (4.6% vs 3.4%), and coronary artery disease (3.0% vs 2.5%) (FIGURE).
“The findings of this analysis of AEs are consistent with previous reports that darolutamide is well tolerated in patients with castration-resistant disease,” lead study author Karim Fizazi, MD, PhD, a medical oncologist at Gustave Roussy and a full professor in oncology at the University of Paris-Saclay in Villejuif, France, said in a virtual presentation of the data during the meeting.
In July 2019, the FDA approved darolutamide for the treatment of patients with nonmetastatic CRPC.2 The approval of the androgen receptor (AR) inhibitor is based on data from the ARAMIS trial, in which darolutamide plus ADT led to a 59% reduction in the risk of metastasis or death vs placebo plus ADT in this patient population (HR, 0.41; 95% CI, 0.34-0.50; 2-sided, P < .0001).3 Moreover, the median metastasis-free survival was 40.4 months with the addition of darolutamide vs 18.4 months with placebo/ADT at a median follow-up of 17.9 months.
In an updated analysis that was performed with a median follow-up of 29.0 months, 83% (95% CI, 80%-86%) of patients in the darolutamide arm were alive at 3 years vs 77% (95% CI, 72%-81%) of patients in the placebo arm. This resulted in a 31% reduction in the risk of death with darolutamide vs placebo (HR, 0.69; 95% CI, 0.53-0.88; P = .003).4
The safety profile of darolutamide was also shown to be favorable, with comparable discontinuation rates because of AEs between the AR inhibitor (8.9%) and placebo (8.7%) in the primary and updated analyses.3,4
To better understand the profile of HTR AEs patients experience on darolutamide, investigators performed an analysis to characterize their onset and occurrence over time vs placebo.
HTR AEs include fatigue, memory impairment, hypertension, falls, fractures, hot flush, gynecomastia, erectile dysfunction, anemia, diabetes, cardiac disorders, weight gain, and dyslipidemia.
HTR AE rates were determined by Common Terminology Criteria for Adverse Events version 4.03, and the cumulative incidences of HTR AEs were evaluated using Kaplan-Meier estimates.
The observation period was discontinued at 2 years to ensure at least 10% of the population was at risk of AEs in each arm.
During the double-blind period, time interval–specific new event rates of HTR AEs were determined for the time between consecutive visits.
Additional findings indicated that memory impairment, a central nervous system AE commonly associated with AR inhibitors, had a lower cumulative incidence in the darolutamide arm vs the placebo arm, at 0.7% vs 1.6%, respectively.
Other HTR AEs that occurred in up to 2% of patients in the darolutamide and placebo arms, respectively, included gynecomastia (1.7% vs 1.1%), heart failure (1.5% vs 0.7%), weight gain (1.3% vs 1.1%), acute myocardial infarction (1.2% vs 0.7%), diabetes (0.9% vs 1.1%), dyslipidemia (0.3% vs 0.2%), and erectile dysfunction (0.1% vs 0.2%). However, Fizazi noted that the cumulative incidences of these HTR AEs were too low to provide time interval–specific analyses.
Moreover, the cumulative incidence of fatigue showed minimal increase over time, and most new events of fatigue occurred during the first month of treatment.
Furthermore, the cumulative incidences of hypertension were similar between treatments; hot flush showed minimal increase over time. New onset of hypertension was not time specific, whereas most new events of hot flush occurred during the first month of treatment with darolutamide.
Falls and fractures had similar cumulative incidences between arms, and most fall and fracture events occurred after the first month of treatment.
The cumulative incidences of anemia and coronary artery disorders were comparable between arms, and new anemia events increased during the first 6 months of treatment and then decreased after 1 year. The new onset of coronary artery disorders was not time specific for darolutamide or placebo.
“Most HTR AEs occurred at a low incidence with darolutamide and with similar incidence to that of placebo. These findings confirm the favorable safety profile of darolutamide,” Fizazi concluded
REFERENCES:
1. Fizazi K, Shore N, Smith M, et al. Hormone treatment–related adverse events (HTR AEs) with darolutamide (DARO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC) from the phase 3 ARAMIS study. Presented at: 36th Annual European Association of Urology Congress; July 8-12, 2021; virtual. Abstract P1226.
2. FDA approves Bayer’s Nubeqa (darolutamide), a new treatment for men with non-metastatic castration-resistant prostate cancer. News release. Bayer. Published July 30, 2019. Accessed July 12, 2021. https://prn.to/3xChOpt
3. Fizazi K, Shore N, Tammela T, et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Eng J Med. 2019;380(13):1235-1246. doi:10.1056/NEJMoa1815671
4. Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040-1049. doi:10.1056/NEJMoa2001342

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More