Selecting and Sequencing Therapy in Advanced Urothelial Carcinoma: Navigating a Dynamic Landscape
Cisplatin-based chemotherapy has been and remains the standard of care in advanced urothelial carcinoma, and can provide a cure for a small subset of these patients.
Abstract
Cisplatin-based chemotherapy has been and remains the standard of care in advanced urothelial carcinoma (aUC), and can provide a cure for a small subset of these patients. The clinical challenge in aUC is that most patients who are treated with cisplatin-based chemotherapy will ultimately develop refractory aUC and succumb to their disease. There is also a large cohort of patients who have other comorbidities that deem them ineligible to receive these regimens. Fortunately, since May 2016, 5 checkpoint inhibitors (CPIs) have been approved for the treatment of aUC. The rapid approval of these agents presents the clinician with challenges navigating drug selection and sequence decisions. We present an overview of the data that resulted in the approval of these agents, and present our approach to the treatment of patients with aUC.
Introduction
Although a substantial percentage of patients with localized urothelial carcinoma (UC) will be cured primarily with local disease control, outcomes for patients with advanced disease remain exceedingly poor. For decades, the standard of care (SOC) for advanced UC (aUC) has been cisplatin-based chemotherapy, which results in a median survival of only approximately 15 months.1Although 13% to 15% of patients with aUC who are treated with chemotherapy can be cured,2most patients ultimately die of their disease.
Recently, novel immunotherapy with checkpoint inhibitors (CPIs) that target programmed cell death-1 (PD- 1) and programmed death ligand-1 (PD-L1) on T cells and tumor cells (TCs), respectively, has revolutionized therapy for patients with aUC. Five CPIs (atezolizumab, pembrolizumab, avelumab, durvalumab, and nivolumab) are now approved by the FDA for patients with aUC with relapsed/refractory disease following platinumbased chemotherapy. Similarly, 2 of these CPIs (atezolizumab and pembrolizumab) have also gained approval for patients with aUC who are cisplatin-ineligible.3-8
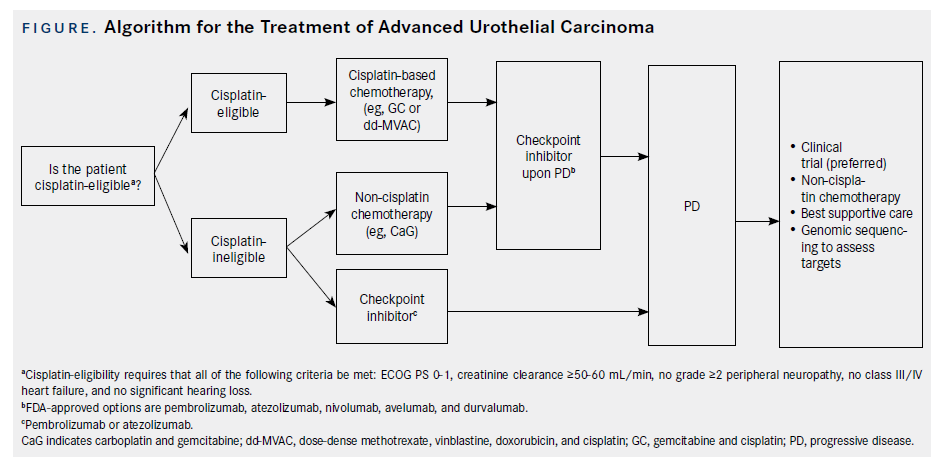
The approval of multiple agents with similar mechanisms of action presents multiple dilemmas for the physician. Most notably, initial drug choice and sequence of therapies in aUC is vague and demands clarification. In this review, we present an overview of the pivotal trials and data that have resulted in the approval of these agents, and present our algorithmic approach for the treatment of patients with aUC.
Chemotherapy
A detailed overview of the role and data for chemotherapy in aUC is beyond the scope of this article. However, a brief overview is critical because cisplatin- based chemotherapy remains a cornerstone of therapy for patients with aUC. The combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) was the historic SOC regimen in aUC, on the basis of results from a phase III trial that demonstrated improved overall survival (OS) with MVAC compared with cisplatin monotherapy (12.5 vs 8.2 months).9Given the relative unfavorable toxicity profile of traditional MVAC, efforts were undertaken to develop less-toxic, cisplatin-based combination therapies for patients with aUC.
Multiple phase II trials demonstrated that the combination of gemcitabine plus cisplatin (GC) was better tolerated than and had similar clinical efficacy to MVAC.10,11As such, a phase III trial comparing GC to MVAC in patients with aUC was conducted, with a primary endpoint of demonstrating the superiority of GC to MVAC.1,2In this trial, 405 patients were randomized in 1:1 fashion to receive either GC or MVAC for a maximum of 6 cycles. The primary objective of OS was similar between the GC and MVAC cohorts (14.0 vs 15.2 months; HR, 1.09 [95% CI, 0.88-1.34]; P = .66), as were the 5-year survival rates (GC,3.0; MVAC, 15.3; P = .53).2Other efficacy endpoints such as objective response rate (ORR), time to progressive disease, and time-to-treatment failure were also similar between the 2 arms. The toxicity profile favored the GC cohort, in which there was less grade 3/4 neutropenia (71% vs 82%), less neutropenic fever (2% vs 14%), and a lower treatment-related mortality rate (1% vs 3%) compared with the classic MVAC cohort.1Although the trial was not designed to demonstrate noninferiority of GC compared with MVAC, many clinicians rely on these data to support the use of GC as a standard frontline therapy for aUC, given similar efficacy and less toxicity.
Other methods of improving toxicity of traditional GC and MVAC, in which the same drugs are given on slightly different schedules, have been thoroughly investigated and incorporated into clinical practice.12,13 Perhaps most notable was the introduction of the high-dose-intensity MVAC (HD-MVAC; also known as dose-dense MVAC [dd-MVAC]) regimen, in which patients are treated with MVAC every 2 weeks (standard dosage of each drug) with granulocyte colony-stimulating factor (G-CSF) support instead of the 4-week traditional (classic) MVAC regimen. In an international phase III trial comparing classic MVAC versus HD-MVAC in aUC, patients receiving HD-MVAC had less toxicity, fewer treatment delays, and were able to receive twice the number of cisplatin doses as the classic MVAC cohort, despite being on a 14-day treatment regimen.12In a long-term follow-up of this study, patients who received HD-MVAC had improved ORR, complete response (CR), and progression-free survival (PFS) compared with those who received classic MVAC.14 Thus, HD-MVAC is a generally accepted standard over the traditional MVAC in this setting.
Despite these dosage and schedule modifications, cisplatin-based chemotherapy remains relatively toxic. A primary challenge, therefore, is determining which patients should be given these chemotherapy regimens. A consensus panel developed guidelines to determine cisplatin eligibility. The consensus guidelines declare a patient as “cisplatin-eligible” if they meet the following criteria: ECOG PS (European Cooperative Oncology Group performance status) 0-1, creatinine clearance (CrCl) ≥60 mL/min, no grade ≥2 peripheral neuropathy, no New York Heart Association Class III/IV heart failure, and no significant hearing loss.15However, the optimal cutoff of adequate renal function is being discussed, with a number of experts administering cisplatin with CrCl ≥ 50 mL/min, often with “split dose” cisplatin (dose divided over 2 days) in borderline cases, while 24- hour urine can be used in such cases to calculate more accurately CrCl. Given the level I (based on randomized phase III trials) evidence of cisplatinbased chemotherapy in aUC and 5-year survival rates of approximately 15%, patients with aUC who meet the eligibility criteria for cisplatin should thus be treated with either GC or ddMVAC as SOC frontline therapy (Figure).
Postchemotherapy Checkpoint Inhibitors in Platinum-Refractory aUC
The Data
Historically, patients who progressed on or following platinum-based chemotherapy for aUC were treated with single-agent chemotherapy such as paclitaxel or docetaxel (or vinflunine in Europe).16However, median PFS and OS with these agents are only approximately 3 and 7 months, respectively, underscoring the need for alternative therapies. Recently, multiple CPIs were approved in platinum-refractory aUC, offering superior clinical options for these patients (Table 1).
The first CPI approved for platinum-refractory aUC was atezolizumab (antiPD-L1). IMvigor 2106was a single-arm phase II trial investigating the use of atezolizumab in patients with aUC. This trial comprised 2 cohorts; cisplatin-ineligible patients (cohort 1); and patients who progressed after platinum- based therapy (cohort 2). In cohort 2, a total of 310 patients were treated with atezolizumab at the standard dosage of 1200 mg every 3 weeks. The primary endpoint of ORR was 15% in all patients. Of note, patients who had increased PD-L1 expression of tumor-infiltrating immune cells (IC0: <1%; IC1: ≥1% and <5%; IC2/3 ≥5%) had higher response rates than those with lower IC expression. IC2/3 patients had ORR of 26%, and IC1/2/3 demonstrated ORR of 18%. Responses were also durable, with 84% of patients having an ongoing response at a median follow-up of 11.7 months. Interestingly, mutational load and The Cancer Genome Atlas (TCGA) luminal II subtype were predictive of higher response rate to treatment. Median OS was 7.9 months in all patients, but was significantly higher in the IC2/3 cohort (11.4 months).6Adverse events (AEs) are noted in Table 1.
Based on these data, on May 18, 2016, the FDA granted accelerated approval for atezolizumab in patients with platinum-refractory aUC. There are a number of interesting features of this approval. In addition to the approval of atezolizumab, the FDA concurrently approved the complementary diagnostic VENTANA PD-L1 (SP142) assay. Importantly, despite its approval as a complementary diagnostic, atezolizumab was approved regardless of PD-L1 expression because of objective and durable responses noted, even with low PD-L1 expression.6 It is also interesting to note that this approval was based on a single-arm trial in the context of historical control data (ie, historical response rates of chemotherapy in the platinum-refractory setting). This highlights the dire need for effective treatments in this setting.
Atezolizumab was granted accelerated approval while the phase III IMvigor 211 confirmatory study of atezolizumab versus chemotherapy was enrolling patients.17A press release by Roche on May 10, 2017, announced that the IMvigor 211 study did not meet its primary endpoint of OS compared with chemotherapy in the predefined patient subset with IC2/3 PDL1 expression.18Although the full data from this trial have not yet been published, atezolizumab retains its accelerated FDA approval for platinumrefractory aUC, and was recently also approved by the European Medicines Agency (EMA).
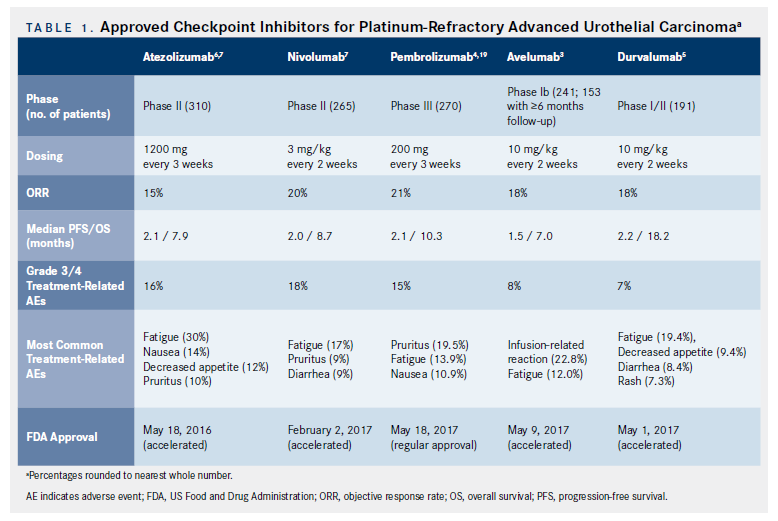
The KEYNOTE-045 trial was a phase III, international trial that randomized 542 patients with platinum-refractory disease to receive either pembrolizumab (antiPD-1) 200 mg every 3 weeks or chemotherapy per investigator choice of docetaxel, vinflunine, or paclitaxel.4,19Median OS (mOS) in the entire cohort, irrespective of PD-L1 expression, was significantly higher in pembrolizumab-treated patients compared with those treated with chemotherapy (10.3 vs 7.4 months; HR, 0.73 [95% CI, 0.59- 0.91]; P = .002). PD-L1 expression was investigated using a combined positive score (CPS), which calculated PD-L1 expression on TCs and ICs relative to the total number of TCs. In patients with a CPS of ≥10, the mOS was superior in the pembrolizumabversus- chemotherapy cohorts (8.0 vs 5.2 months; HR, 0.57 [95% CI, 0.37-0.88]; P = .005).4 Treatmentrelated AEs were also less common in the pembrolizumab arm (Table 1). In an updated analysis, ORR was 21.1% for pembrolizumab versus 11.0% for chemotherapy- treated patients. The median duration of response (DoR) was not reached for pembrolizumab (1.6 - 20.7+) and was 4.4 months (1.4-20.3+) for the chemotherapy cohort.19Pembrolizumab thus received regular approval for patients with platinumrefractory aUC, and was also approved by the EMA. In addition to the clinical trials of pembrolizumab and atezolizumab, which have data regarding their phase III trial results either in press release and presented abstract (atezolizumab) or academically published (pembrolizumab) form, 3 other CPI agents are currently approved in platinum-refractory aUC. These agentsnivolumab (antiPD-1), avelumab (anti–PD-L1), and durvalumab (anti–PD-L1)—are all approved based on results from single-arm phase I/ II clinical trials3,5,20,21(Table 1).
Choosing an Immunotherapy in Platinum-Refractory aUC
In the now-crowded platinum-refractory aUC setting, choosing between CPI options has not been clearly defined. Our approach is, therefore, not based on rigorous head-to-head comparisons of agents, but rather based on the current and emerging level of evidence supporting these agents. Nivolumab, durvalumab, avelumab, and atezolizumab are approved on the basis of phase I/II data. Moreover, the IMvigor phase III trial data for atezolizumab was reported as not having met its primary endpoint. However, pembrolizumab is approved on the basis of the results from a phase III clinical trial. Given the superiority of this level of evidence, it is reasonable to use pembrolizumab in platinum-refractory aUC. However, any of the above agents are appropriate based on FDA approval, and all 5 appear to have comparable efficacy and safety profiles, although any cross-comparisons between clinical trials have inherent limitations. Clinical trials remain a very critical part of new treatment evaluation and should always be considered.
Frontline Cisplatin-Ineligible
The Data
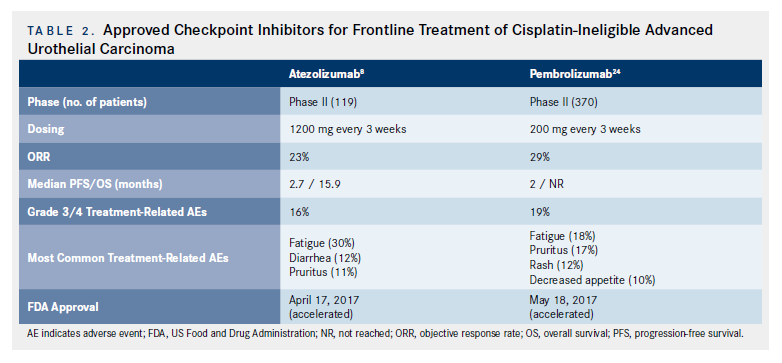
Historically, patients who were not clinically fit to be treated with cisplatin were treated with other chemotherapy regimens (eg, carboplatin-based chemotherapy), which, although active, are significantly less effective than cisplatin.22,23More recently, however, that paradigm has shifted as CPIs have been incorporated into first-line therapy for cisplatin- ineligible patients with aUC, based on results of phase II, single-arm clinical trials (Figure; Table 2).
As previously mentioned, IMvigor 210 was a single-arm, 2-cohort phase II trial investigating the use of atezolizumab in patients with aUC.8In cohort 1 (cisplatin-ineligible patients), 119 patients were treated with atezolizumab 1200 mg every 3 weeks until progression. With a median follow-up of 17.2 months, the ORRs and CRs were 23% and 9%, respectively, with a median OS of 15.9 months (10.4-not reached [NR]). At a median follow-up of 17.2 months, 70% of responses were still ongoing.8Immune-mediated AEs occurred in 12% of patients. Interestingly, higher mutational load was associated with higher response rates.8These impressive efficacy and safety results in this setting led to accelerated FDA approval of atezolizumab as a frontline treatment option for cisplatin-ineligible patients with aUC, and atezolizumab also was recently approved by the EMA
KEYNOTE-052 was a single arm, phase II trial of pembrolizumab in the first-line setting for cisplatinineligible patients.24These 370 patients were treated with pembrolizumab 200 mg every 3 weeks for a maximum of 24 months or until progressive disease (PD), intolerable toxicity, or withdrawal of consent. With a median follow-up of 9.5 months, the primary endpoint of ORR was 29% (7% CR). The median time to response was 2 months (range, 1-9 months). Median DoR was not reached (12 months NR). Impressively, 82% of responses lasted more than 6 months.24Adverse events are highlighted in Table 2. Efficacy and safety appear similar, even in the subset of patients with poor PS and in the elderly (age ≥75 years). Based on these data, the FDA granted accelerated approval to pembrolizumab in cisplatin-ineligible aUC, and it was also recently approved in Europe.
Choosing Therapy in Cisplatin-Ineligible Patients
Despite advances with CPIs, it is important to note that patients who are cisplatin-ineligible may still be treated with other chemotherapeutic agents, such as carboplatin-based regimens (Figure).22,23However, patients who are generally unfit for any chemotherapy due to medical comorbidities, poor performance status, or other reasons may receive CPI therapy as SOC. Similarly, genomic sequencing of circulating tumor DNA and tumor tissue can be used to guide therapy (Figure).25Clinical trials are an important option, as well, and should be considered in the appropriate setting.
With regard to CPI treatment options in cisplatinineligible and chemotherapy-ineligible patients with aUC, the efficacy of pembrolizumab and atezolizumab appears comparable, taking into account the limitations of cross-trial comparisons (Table 2). The level of evidence is also identical as both trials were single-arm, noncomparator, phase II trials. Therefore, both are appropriate agents in this clinical setting. An institution may implement a policy in which a specific CPI is on formulary based on other cancer types and/or treatment settings (eg, data as noted above regarding pembrolizumab in platinum-refractory aUC). Such institutional selection, available guidelines, insurance policies, frequency of administration, and costeffectiveness studies in the “real world” setting may ultimately guide therapeutic choice of CPI in different settings. Importantly, however, from an evidence-based approach, both atezolizumab and pembrolizumab are acceptable agents in the cisplatin-ineligible, frontline aUC setting.
Conclusions
Chemotherapy has been the cornerstone of aUC management and still has an established role, especially cisplatin-based chemotherapy in the first-line setting. A number of clinical trials in this setting are comparing chemotherapy to immunotherapy, combinations of chemotherapy and immunotherapy, or sequential strategies such as switching to maintenance immunotherapy after an initial response or stable disease to chemotherapy. The emergence of CPIs in both cisplatin-ineligible and platinumrefractory aUC has resulted in a much-needed improvement in clinical outcomes in a number of patients; however, more progress is urgently and clearly required. Pembrolizumab and atezolizumab are options in the cisplatin-ineligible setting, with pembrolizumab owning the highest level of evidence in platinum-refractory disease.
We acknowledge that all of the agents mentioned above are FDA approved and appropriate for clinical practice in the appropriate setting. Of note, there is a plethora of clinical trials as well as translational We acknowledge that all of the agents mentioned above are FDA approved and appropriate for clinical practice in the appropriate setting. Of note, there is a plethora of clinical trials as well as translational research investigating the molecular biology of UC (eg, germline and somatic mutations, gene expression profiling, mutational load, PD-L1 expression, other biomarkers).6,24,26,27Our hope and anticipation is that these research endeavors will ultimately drive treatment selection and further improve outcomes for patients with aUC.
References
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study.J Clin Oncol.2000; 18(17):3068-3077.
- von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer.J Clin Oncol. 2005; 23(21):4602-4608.
- Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase ib study.J Clin Oncol.2017;35(19):2117-2124. doi: 10.1200/JCO.2016.71.6795.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as secondline therapy for advanced urothelial carcinoma.N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683.
- Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study.JAMA Oncol.2017;3(9):e172411. doi: 10.1001/jamaoncol.2017.2411.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial.Lancet. 2016;387(10031):1909- 1920. doi: 10.1016/S0140-6736(16)00561-4.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial.Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7.
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial.Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2.
- Loehrer PJ S, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study.J Clin Oncol. 1992; 10(7):1066-1073.
- von der Maase H. Gemcitabine in locally advanced and/or metastatic bladder cancer.Crit Rev Oncol Hematol. 2000; 34(3):175-183.
- von der Maase H, Andersen L, Crino L, Weinknecht S, Dogliotti L. Weekly gemcitabine and cisplatin combination therapy in patients with transitional cell carcinoma of the urothelium: a phase II clinical trial.Ann Oncol. 1999; 10(12):1461-1465.
- Sternberg CN, de Mulder PH, Schornagel JH, et al; European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer protocol no. 30924.J Clin Oncol.2001; 19(10):2638-2646.
- Als AB, Sengelov L, Von Der Maase H. Gemcitabine and cisplatin in locally advanced and metastatic bladder cancer; 3- or 4-week schedule?Acta Oncol. 2008; 47(1):110-119.
- Sternberg CN, de Mulder P, Schornagel JH, et al; EORTC Genito-Urinary Cancer Group. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours.Eur J Cancer.2006; 42(1):50-54.
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy.J Clin Oncol.2011;29(17):2432-2438. doi: 10.1200/JCO.2011.34.8433.
- Raggi D, Miceli R, Sonpavde G, et al. Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis.Ann Oncol. 2016;27(1):49-61. doi: 10.1093/annonc/mdv509.
- A study of atezolizumab compared with chemotherapy in participants with locally advanced or metastatic urothelial bladder cancer [IMvigor211]. clinicaltrials.gov/ct2/show/NCT02302807. Updated December 13, 2017. Accessed December 10, 2017.
- Roche provides update on phase III study of TECENTRIQ (atezolizumab) in people with previously treated advanced bladder cancer [press release]. www.roche.com/media/store/releases/med-cor-2017-05-10.htm. Basel, Switzerland: Roche; May 10, 2017.
- Bajorin DF, DeWit R, Vaughn DJ, et al: Planned survival analysis from KEYNOTE-045: phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC).J Clin Oncol.2017; 35(suppl; abstr 4501).
- Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab(MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer.J Clin Oncol. 2016;34(26):3119-3125. doi: 10.1200/JCO.2016.67.9761.
- Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multi-arm, phase 1/2 trial.Lancet Oncol.2016; 17(11):1590-1598. doi: 10.1016/S1470-2045(16)30496-X.
- Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23(2):406-410. doi: 10.1093/annonc/mdr156.
- De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986.J Clin Oncol. 2012;30(2):191-9. doi: 10.1200/JCO.2011.37.3571.
- O’Donnell PH, Grivas P, Balar AV, et al: Biomarker findings and mature clinical results from KEYNOTE-052: first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC).J Clin Oncol. 2017;35(suppl; abstr 4502).
- Barata PC, Koshkin VS, Funchain P, et al. Next-generation sequencing (NGS) of cell-free circulating tumor DNA and tumor tissue in patients with advanced urothelial cancer: a pilot assessment of concordance.Ann Oncol. 2017;28(10):2458-2463. doi: 10.1093/annonc/mdx405.
- Carlo MI, Zhang L, Mandelker D, et al. Cancer predisposing germline mutations in patients (pts) with urothelial cancer (UC) of the renal pelvis (R-P), ureter (U) and bladder (B).J Clin Oncol. 2017;35(suppl; abstr 4510).
- Lerner SP, Roberton G, Kim J, et al. Comprehensive molecular characterization and analysis of muscle-invasive urothelial carcinomas.J Clin Oncol. 2017; 35(suppl 15; abstr 4500).














2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.

