Roundtable Discussion: McKay Covers First-Line Decision-Making for Metastatic RCC
During a Targeted Oncology case-based roundtable event, Rana McKay, MD, and participants discussed the case of a patient with previously untreated advanced renal cell carcinoma with favorable risk status.
Rana McKay, MD
Associate Professor of Medicine
UC San Diego Health
San Diego, CA


CASE SUMMARY
The patient is a 61-year-old man, married, and the father of 2 grown children, with 5 grandchildren who live nearby. He also lives an active lifestyle (takes daily walks and golfs regularly). He has a history of metastatic renal cell carcinoma and 4 years later, his recurrence of lung nodules with biopsy are consistent with clear cell renal cell carcinoma (RCC). In retrospect, the nodules had been present on scans for at least 2 years prior. The patient was observed based on low volume and indolence of disease and patient preference. Eighteen months later, he continued indolent growth on scans, increased total tumor burden, and a new paratracheal lymph node (2.0 × 1.5 cm) was observed. His ECOG performance status was 0.
DISCUSSION QUESTIONS
- What additional workup do you typically order?
- Would you initiate systemic therapy at this point?
MCKAY: What would you do for additional diagnostics, and would you initiate treatment?
KOSIEROWSKI: I would check an MRI of the brain before I make that decision.
MCKAY: Great, any other thoughts? Would anybody start treatment on this gentleman? Let’s say you get the MRI, and the MRI is negative.
KOSIEROWSKI: I might continue to watch him for a little longer.
GOLDBERG: It’s a tough decision to make. You don’t want him to have too much bulky disease. He’s in the hinterland. I agree with the previous comment, but I don’t know. I’d have to look at the whole picture. [I’d look at] how many nodules he [has]. The disease is slowly progressing. He’s had a long disease-free interval, although it seems, in retrospect, it was 2 years rather than 4 years. But I could go either way. If you could get a good response with a biologically slowly growing tumor, you might buy more time at the end. But again, I wouldn’t argue with either way.
MCKAY: What are the factors you all think about when you’re making the decision about treatment? I heard some of you saying number of nodules [and] disease burden. What are the things you all look at when deciding to treat [patients]?
GOLDBERG: I think the biggest thing is quality of life.
KOSIEROWSKI: I’ll add comorbidities. How likely is the patient to die of something else intercurrently? This patient we are discussing looks well, so I don’t think that’s part of [the decision-making for him]. But certainly, comorbidities will determine when and [whether] we start therapy, [as well as] what therapy we’re going to use.
CHARU: The bulk of disease will also be important. If he has extensive disease, then one might want to consider treatment.
MCKAY: This is a favorable-risk patient with favorable-risk RCC, and sometimes they can be the most challenging because it’s not so clear cut.
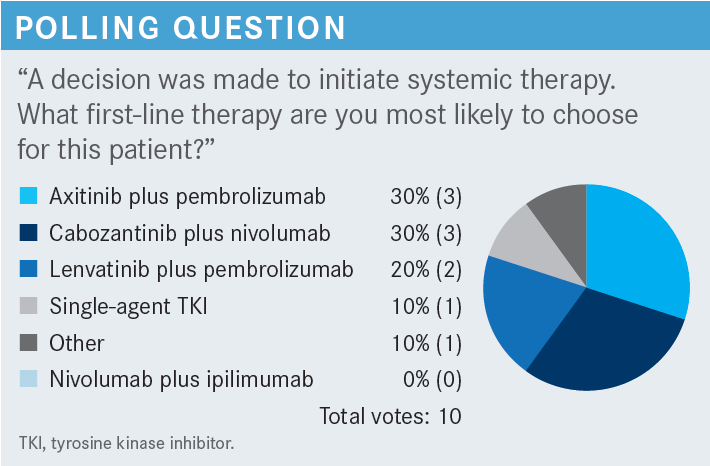
CHEN: I chose a combination of either axitinib [Inlyta] plus pembrolizumab [Keytruda; axi-pembro] or cabozantinib [Cabometyx] plus nivolumab [Opdivo; cabo-nivo]. For the patients [described in the poll scenario], I feel that the combination of the tyrosine kinase inhibitor [TKI] and the anti–PD-1 drug [would] have a good response rate and would work well in both situations.
MCKAY: Yes, the data are strong for immuno-oncology [IO] plus VEGF [inhibitor] combinations. Anybody else? What about the individual who had selected single-agent TKI therapy and switched to something else [in the second poll scenario]?
AGARWAL: I chose the single agent because the patient is relatively asymptomatic, with very indolent disease over years. With more than 2 visceral sites of disease, I would want to give them the combination.
DISCUSSION QUESTIONS
- How do you assess risk?
- Does risk status influence your first-line decision-making for a patient like this?
- What are the primary factors that drive your selection of first-line therapy for patients with favorable-risk metastatic clear cell RCC?
- Balance of efficacy, quality of life, and safety
- Is progressive disease important to you in terms of clinical decision-making?
- Sites of metastasis
- Number of sites of metastasis
- Patient preference
- Other
- What about for patients on the borderline of favorable vs intermediate risk? What influences your first-line recommendations?
MCKAY: I’d love to hear how you assess risk in the clinic. Are you doing International Metastatic RCC Database Consortium risk stratification? If not, what else are you using? How does risk stratification influence your decision around first-line treatment, particularly for a patient like the one we presented?
ZHOU: I don’t exactly go with the risk stratification. The reason I don’t is because my go-to regimen is always the combination, whether the disease is low risk or high risk. That’s my practice.
ANAND: I use the risk stratification, but I don’t think it helps me guide treatment. I use it just to know [the risk status]. The treatments are not that different for favorable- vs poor-risk disease. I always use a combination, and I’ve had good experience with cabozantinib plus nivolumab as far as tolerance, because we use the intermediate dose of cabozantinib, and I feel like patients have fewer adverse events [AEs]. I’ve had some bad experience with lenvatinib [Lenvima] as far as [adverse] effects. I don’t think it’s very well tolerated. That’s been my limited experience, so I tend to use cabozantinib plus nivolumab.
GOLDBERG: I think a lot depends on presentation [of the disease]. Has the patient already had a nephrectomy? It also depends on the disease-free interval. Are they presenting with widespread and metastatic disease off the bat?
I think clinical impression is more important than using guidelines for assessing risk. If one worries about [the difference] between intermediate and favorable risk, wait 3 months or longer and see what’s happening. But I just think it’s presentation that is important, [as well as] bulk of disease.
MCKAY: There are a lot of recurring themes here about presentation, bulk of disease, tumor burden, and sites of metastasis. I certainly know that no risk stratification tool is perfect. Some of them use lab abnormalities, and if a patient has some chronic kidney disease because they had a nephrectomy, and they have a little mild anemia, are they truly intermediate risk? What is their status? Teasing that out is key. What are other [factors] that drive your decision-making around first-line or second-line therapy?
CHEN: I do use risk stratification, but I agree with everyone else that clinical presentation is important. A patient who comes in with a brain metastasis is not going to be a low-risk patient. [Often], the clinical presentation probably drives the decision-making in terms of how fast we need to start treatment and what treatment we use.
MCKAY: What about those patients [who] are borderline between favorable and intermediate risk? Does anything sway you one way or another? It seems like most are leaning toward IO plus VEGF inhibitor combinations for favorable-risk patients. Does anybody ever consider an IO-IO combination for these individuals, knowing the durability of those regimens?
CHEN: I’ve used IO-IO combinations, but I’ve also run into more problems with immunotherapy adverse effects with these combinations. I had [a] patient who developed adrenal problems [as an] immune-related [adverse] effect. Another patient developed a hepatotoxicity with IO-IO therapy. I see, certainly, that there are more of the immune-related AEs.
MCKAY: Do you feel, in your practice, that there’s a differential [adverse] effect pattern in the favorable- vs intermediate- and poor-risk patients, or not really?
CHEN: I don’t think I’ve teased that out yet.
ZHOU: I do [have the] concern that immunotherapy [AEs] are sometimes [more] difficult to deal with. [With other] medication, if you have AEs, you can hold off the medication or reduce the dose. It’s much easier to treat.
MCKAY: Has that been everybody’s general experience, that IO-IO combination therapy is more challenging than IO plus VEGF inhibitor therapy?
YEH: I think the TKI has got quite a bit of toxicity, particularly upper gastrointestinal toxicity, diarrhea, and myelosuppression. Neutropenia is also common, so you may have to dose adjust. The TKI itself is quite toxic, and that’s my experience, [at least].
MCKAY: Well, this has been very enlightening, to think about the different factors that weigh in and what your experiences have been around treating favorable-risk patients with combinations of either IO-IO or IO plus VEGF inhibitor therapy.
CASE UPDATE
Cabozantinib (40 mg daily) plus nivolumab (240 mg every 2 weeks) was initiated.
DISCUSSION QUESTIONS
- What are your perspectives on the CheckMate 9ER (NCT03141177), KEYNOTE-426 (NCT02853331), and CLEAR (NCT02811861) study data?
- When you think about the immune checkpoint inhibitor plus TKI combination regimens for favorable-risk metastatic RCC, what differentiations come to mind? Thinking about each combination, what patient profile comes to mind?
- Please discuss your real-world experience with each immune checkpoint inhibitor plus TKI regimen (axi-pembro, cabo-nivo, and lenvatinib plus pembrolizumab [len-pembro]). How do the safety profiles of the regimens compare?
- Thinking about each regimen, how often do you have to modify, interrupt, or discontinue treatment because of toxicity?
- To what extent does/should the TKI guide treatment selection? How much consideration do you give to the TKI included in each regimen?
MCKAY: I would love to hear what you think of these data. How do you think the data apply to the favorable-risk population? What’s been your real-world experience with [using] these drugs in the clinic? Let’s open the floor for discussion.
KOSIEROWSKI: At least with the IO-TKI combinations, you look at the IO component first, and I don’t think there’s much difference between them. I think the dosing frequency is different [for] pembrolizumab [vs] nivolumab, and that can be a good factor. However, I think most of the toxicities we see come from the TKI part, and that’s going to be individual, but you’re going to identify that toxicity relatively quickly. So, I just go with my first go-to [choice], then I modify [from] there, looking mainly at the toxicity from the TKI. If possible, I like to use the longer-acting IO.
MCKAY: Thanks for sharing that. Does anybody else want to share their experience about their real-world practice with these regimens and what these data highlight?
CHARU: I have issues using lenvatinib sometimes. The patients don’t tolerate it well, so my go-to regimen is [often] cabo-nivo.
MCKAY: Do others have that experience with lenvatinib? When you use lenvatinib, do you start at the 20-mg dose when you’re using it with pembrolizumab?
STEPHEN: I use len-pembro with good effectiveness in different cancer types, so I’m used to it. But I tend to start with the [recommended] renal dose, or maybe a little below the recommended dose, and then maybe increase the dose if the patient is tolerating it well. I am quick to dose reduce based on hypertension; I think that’s one of the main AEs. Being vigilant about it, I’ve had good luck with [that combination], but I was interested to see the data that showed the quality of life was better with the cabo-nivo combination,1 so maybe I should be [using] that more.
CHEN: When I use lenvatinib, I also start with a lower dose— approximately 12 mg instead of starting at 20 mg or 18 mg— then I go up as tolerated. Typically, I start at 12 mg. For cabozantinib, I’m curious to see what most physicians start with. Do they start with the 40-mg dose, or do they usually go even a little lower than 40 mg to start?
CHARU: I have started with 40 mg. I didn’t have many issues.
MCKAY: I was going to highlight that it’s sometimes hard to interpret quality-of-life data, because the time point at which the questionnaires were deployed was different across the 3 trials, and the instruments were all slightly different. Whether [the patients] had symptomatic disease at baseline was also different. The percentage of patients [who] were favorable vs poor risk differed across the studies. If patients have poor-risk disease and they’re very symptomatic from their cancer, an effective therapy is going to improve their quality of life [From the Data1]. We see that in the cabo-nivo data.1,2

With the axi-pembro3,4 and len-pembro5,6 [studies], there were more favorable-risk patients enrolled. The other interesting piece about the cabozantinib data is that in the sunitinib arm, patients had to fill out the questionnaires after the 2-week hold on sunitinib. It’s interesting that the data looked as good as they did, because in the control arm, patients were filling out the survey after having a 2-week holiday from their TKI.1 I think [those] data [are] compelling. Are there any other thoughts from the group about the regimens [or] the safety profiles?
GOLDBERG: I agree with you that response rate usually makes the patients feel better, not only physically but also mentally, when they’ve been told [their] test is [reporting] a smaller amount of cancer. One has to weigh the quality of life with the response rate, and it’s difficult to measure. Quality of life [is] almost in the eyes of the beholder [in] discussion with the patient.
In my opinion, you go with the best statistics, and everything is compared with sunitinib, which [almost] no one uses anymore. It’d be wonderful to see a study between the 3 big [combinations—axi-pembro, cabo-nivo, and len-pembro]— which will never be done but would make more sense. But I get influenced a little by the complete response rate and the length of progression-free survival [PFS] with the [lenvatinib] over the others.2,3,5 But is there a difference? I’m not sure.
DISCUSSION QUESTIONS
- For this patient who received first-line cabozantinib plus nivolumab, what would you offer as next-line therapy?
- How likely are you to change your practice, with respect to first-line decision-making for patients with favorable-risk metastatic RCC?
KOSIEROWSKI: I assume [the patient] stopped [first-line treatment because of] disease progression and not toxicity?
MCKAY: [Right], not due to toxicity.
GOLDBERG: I’d go to lenvatinib. Whether to add pembrolizumab to it, I’m not sure, but that’s what I would go to for second line.
MCKAY: As a single agent? Or would you do it with everolimus [Afinitor]?
GOLDBERG: Either-or. Probably as a single agent.
MCKAY: Any other thoughts?
ZHOU: This is the frustrating part. When I move to the second line, I tend to choose a different class of medication, but [the choice is] still limited. It’s very frustrating.
MCKAY: Has anybody used tivozanib [Fotivda]?
KOSIEROWSKI: No. I guess the other thing [to consider] is [whether] this is primary-refractory disease or disease recurrence at this point.
CHO: I have used tivozanib. I think it’s a tolerable TKI.
ZHOU: I have a patient with whom I used [everolimus], but I don’t know [whether] anybody here has used it.
MCKAY: If you are likely to change your practice, what was it in the discussion that made you come to that conclusion?
YEH: I think it’s the survival benefit. Regardless of which TKI you use, the overall survival is identical. You know [there will] be a survival benefit, and you know PFS may be a little better [with] lenvatinib, because it has probably the best HR.2,3,5 But overall, the risk-benefit [balance] is similar across the 3 big combinations. I don’t care which one [I use]. If the insurance would pay for it, I would use it.
MCKAY: During our discussion, there seemed to be some [consideration of] cabo-nivo [regarding] the quality-of-life data.1 Are there any other thoughts?
KRIJANOVSKI: It depends on [where] the metastases are and on how much [tumor] burden there is. I guess cabozantinib could also be given at the lower, every-other-day dose, if necessary. But if metastases are in the bones, with or without metastases in the liver, that’s my first choice. In terms of sequencing, I do try to change the [drug class]. I go from immunotherapy to a VEGF inhibitor, and if the patient had immunotherapy up front, I go to a VEGF inhibitor by itself [or in] combination. I have found that patients on dialysis do well with ipilimumab [Yervoy] plus nivolumab. They do have a nice response and better tolerance.
REFERENCES
1. Cella D, Motzer RJ, Suarez C, et al. Patient-reported outcomes with first-line nivolumab plus cabozantinib versus sunitinib in patients with advanced renal cell carcinoma treated in CheckMate 9ER: an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(2):292-303. doi:10.1016/S1470-2045(21)00693-8
2. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
3. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(suppl 15):4500. doi:10.1200/JCO.2021.39.15_suppl.4500
4. Bedke J, Rini B, Plimack E, et al. Health-related quality-of-life (HRQoL) analysis from KEYNOTE-426: pembrolizumab (pembro) plus axitinib (axi) vs sunitinib for advanced renal cell carcinoma (RCC). Presented at: 35th Annual European Association of Urology Congress; July 17-19, 2020; virtual. Accessed November 8, 2022. https://bit.ly/3EQ9poE
5. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
6. Motzer R, Porta C, Alekseev B, et al. Health-related quality-of-life outcomes in patients with advanced renal cell carcinoma treated with lenvatinib plus pembrolizumab or everolimus versus sunitinib (CLEAR): a randomised, phase 3 study. Lancet Oncol. 2022;23(6):768-780. doi:10.1016/S1470-2045(22)00212-1

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen