Roundtable Discussion: Lee and Participants Debate Frontline Therapy in Intermediate-Risk RCC
A 59-year-old Black woman received a diagnosis of clear cell renal cell carcinoma. Chung-Han Lee, Md, PhD and a group of other physicians discussed the patient's case during a Case-Based Roundtable event.
Chung-Han Lee, MD, PhD

During a Targeted Oncology Case-Based Roundtable event, Chung-Han Lee, MD, PhD, a medical oncologist at Memorial Sloan Kettering Cancer Center in New York, NY, discussed that case of a 59-year-old patient with intermediate-risk renal cell carcinoma (RCC).

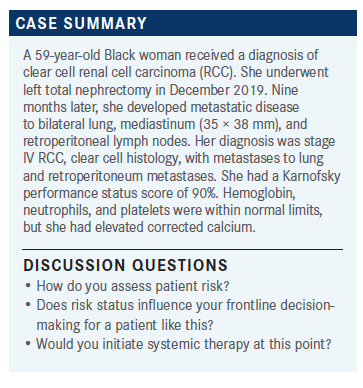
LEE: How would you assess the risk category for this patient? This is open to anyone who wants to go through the International Metastatic RCC Database Consortium risks.
GILLANI: I think this is intermediate risk. There are at least 2 adverse risk factors: time from diagnosis to treatment being less than 1 year and the corrected calcium, which is elevated. So I think this is intermediate risk with 2 unfavorable factors.
LEE: Two unfavorable factors definitely puts them into the intermediate-risk category. For patients like this, what do you think of for treatment? How do you use risk stratification for treatments?
GILLANI: In my mind, the decision is a little bit harder for patients with good- or intermediate-risk disease. I think the choices can be close observation, which is appropriate for a small subset of patients. If they’re asymptomatic, it’s a hard sell to put them on an immunotherapy/tyrosine kinase inhibitor [IO/TKI] combination. If I were to choose something and the patient desires treatment, options range from axitinib [Inlyta], pembrolizumab [Keytruda], to cabozantinib [Cabometyx] and nivolumab [Opdivo] to the newest addition of lenvatinib [Lenvima] and pembrolizumab. Then you also have the option of ipilimumab [Yervoy]/nivolumab. Of all the choices available, I think based on the new data, I might start with dose-modified lenvatinib and pembrolizumab and see how they tolerate it.
LEE: Does the dose modification of the lenvatinib [make a difference in your treatment]? What would you lean toward as a starting dose?
GILLANI: I’m not sure that starting at the 20-mg dose is the smartest thing to do, especially if somebody is frail or older. I might be inclined to start at 10 mg and quickly dose escalate. I know this is not data driven, but I like to see how they tolerate it and then quickly go up on the dose.
LEE: Anyone else have other ways that they would manage this patient?
MUNDIA: The clinical comorbidities are also very important. This patient doesn’t seem to have any autoimmune disease. This is an African American woman. But I’ve seen a number of patients that are in the same age category, and then they have active systemic lupus erythematosus or something like that. I think that makes the IO combination more difficult, and I wouldn’t offer it. I’d start the discussion with this woman with ipilimumab/nivolumab. [This combination has] had pretty good tolerability in my experience and I think the data are pretty outstanding. I’m in community practice, so coverage with the TKI/IO combinations really matters.
Often, I’ve found that the TKI component can have a significant cost sharing or burden of cost to a patient. She’s 59 years old; [if she is] commercially insured, I’m less worried. But if this woman were 66 years old and had Medicare and not a second or managed Medicare, then I think that TKI component would become very difficult. I would start the discussion with this patient with IO/IO and then explore the IO/TKI combinations. I agree with my colleague that if I’m going to use lenvatinib up front— and I haven’t used it up front; I’ve used it ostensibly in the second line—I would start at a much lower dose. I’m concerned about tolerability.
LEE: What dose would you start at?
MUNDIA: I would say 10 mg is probably fair.
LEE: Anyone else have different thoughts on treatment of this patient?
GILLANI: I think I failed to mention that single-agent cabozantinib would not be unreasonable. Similarly, single-agent pazopanib [Votrient] or even sunitinib [Sutent] would not be totally wrong. I know the efficacy is lower, but I think it’s a reasonable choice in a certain subset of patients.
LEE: What types of patients have you been selecting for single-agent TKI?
GILLANI: [I use it] especially in a subset where I’m concerned about IO toxicities or I want to see how they tolerate one agent before I tag along a second drug. If somebody has predominantly bone-only disease, then I might be inclined to use cabozantinib.
ILYAS: As far as the single-agent TKI, in my practice, I would typically prefer [to use it in patients with] favorable risk and limited disease burden. That may be a very reasonable approach—using single-agent oral therapy for those patients.
LEE: What would your TKI choice be for that population?
ILYAS: Cabozantinib, pazopanib, and axitinib are very easy to dose modify, so any of those single agents would be fairly reasonable for that patient population.
GORUSU: I would have gone with the combination of ipilimumab/nivolumab.
LEE: What makes you lean toward ipilimumab/nivolumab?
GORUSU: If I recall, this combination has been approved for both the intermediate- and high-risk settings. I’m following the guidelines. Dosing-wise, after evolving from the melanoma doses vs the lung doses, I am quite comfortable with the dosing of ipilimumab/nivolumab—the way that it is given and that they tolerate it well and it is efficacious. So, probably a component of that is the comfort level; I can handle the patients quite well.
But regarding what my other colleagues were discussing—which dose do I go with, if at all—with [drugs] like lenvatinib, I would have gone with something around 8 or 10 mg. That hassle of worrying about the dose toxicities, even with the cabozantinib, it’s an excellent drug, but can everybody tolerate the 40 mg? Or maybe they just need 20 mg or alternating 20 mg with 40 mg. It is an autopilot [regimen if] the patients tolerate the treatment well. Of course, they’re getting the efficacy as well. That would probably have been my first choice with this patient.
LEE: How often do you or for how long do you typically continue the nivolumab?
GORUSU: [I give it] as long as they are tolerating it and they’re continuing the response.
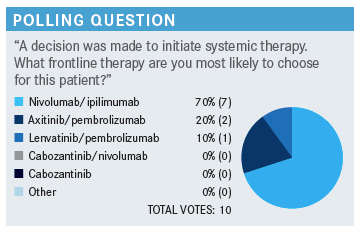
LEE: For the people who picked ipilimumab/nivolumab as the frontline option, what made you pick that for this patient?
REZNIKOFF: I’m not familiar with all the data on all these, but I think it can provide long-term disease-free [survival], maybe longer term than the others. I’m not sure, but I’ve had that experience with other malignancies, and I’m comfortable with it.
ILYAS: I picked ipilimumab/nivolumab. One of the reasons that would have swayed me to lenvatinib and pembrolizumab is if the patient had significant disease burden, life-threatening disease burden, and…we need to get the response as soon as possible. That would have been maybe a better choice [in that case]. I believe that that combination has much higher response rates. For this particular patient, none of these combinations would be wrong, but I picked ipilimumab/nivolumab too for the progression-free [survival] as well as overall survival and the comfort level with this regimen.
LEE: For the people who picked any of the TKI/IO combinations, what made you choose that as the up-front therapy?
GILLANI: We always say that cross-trial comparisons are not a good thing to do. But I think we are led to believe that the latest regimen of lenvatinib and pembrolizumab seems to be the most efficacious regimen in terms of response rate, durability of response, overall survival, etc.…I can understand why people would pick ipilimumab/nivolumab, because the medical oncologist has to manage TKI toxicities, monitor the blood pressure closely, and look for other toxicities vs when doing 2 intravenous drugs. We have become very comfortable with the IO-related toxicities, and I think managing those is not that difficult now. I think that’s a very attractive regimen.
NEWSOME: Also, the ipilimumab falls off, so you’re dealing with single-agent nivolumab; it’s once a month and it’s easy to give. So that’s always tempting—although I must say, I’ve had a couple of patients with melanoma who received ipilimumab/nivolumab and have developed myocarditis. So that’s always humbling. Although it is rare, when you see it, you always have a little reluctance to try it [again]. But ipilimumab/nivolumab tends to be my go-to, for that reason. It’s easy, it’s once a month, and ipilimumab falls off.
LEE: For your patients, have you been using the 2 different dosings for ipilimumab/nivolumab, depending on melanoma vs RCC, or have you been using the same dose for each?
NEWSOME: I’ve generally stuck with the standard dosing for each of them.
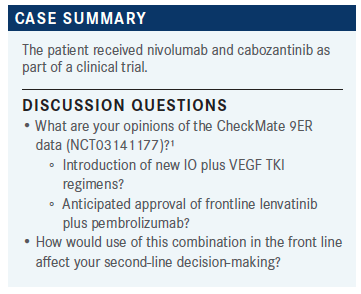
LEE: What were your experiences with or reactions to the nivolumab/cabozantinib data? No one chose that as their preferred TKI/IO regimen.
DESHPANDE: I was very impressed with the data. It seemed to score in all fronts: the response rate, the survival, and the toxicities. I was a little bit surprised because I also treat patients with thyroid cancer and maybe the dose is higher for cabozantinib but it’s a [tough] drug in terms of adverse effects at the 140-mg dose.
GILLANI: I recently had a patient on nivolumab/cabozantinib. I think we went from axitinib/pembrolizumab to nivolumab/cabozantinib to lenvatinib within a span of a few months [and then] nivolumab/cabozantinib. I had an experience similar to what Dr Deshpande was saying—that cabozantinib is a difficult drug to tolerate. [There’s] a lot of asthenia, mucositis, and dysgeusia. I think this patient had a lot of arthralgias as well, and I had to drop the dose quickly to 20 mg. I’m still waiting to see what kind of response he would get from this combination.
MUNDIA: I use a much lower dose of cabozantinib in the combination than I do when I use monotherapy. I think that’s contributed to an improved tolerability, to the point that even when I use monotherapy cabozantinib, I start at 40 mg in some patients.…I think with the approval of lenvatinib, the response rate is so impressive that if I’m going to use a TKI/IO combination, I feel that’s probably— not a scientific comparison—the most efficacious TKI/IO. It doesn’t hurt that pembrolizumab is now every 6 weeks and nivolumab is every 4 weeks. That’s part of that discussion with the patient. I think they’re all equally impressive. When there’s an access issue, I will default to what is easily more affordable for that patient.
LEE: The nivolumab/cabozantinib combination was FDA approved on the heels of axitinib/pembrolizumab and axitinib/avelumab. Were the data sufficient to change anyone’s opinion about the TKI/IO combination of choice—let’s say, prior to the lenvatinib/ pembrolizumab data?
GILLANI: I think it just reinforced that the combination of TKI and IO is active and a very reasonable combination to use. The subset of patients with bone metastases, where there was higher activity, was also a selling point for patients who have predominantly bone-only disease.
LEE: Now with the lenvatinib/pembrolizumab data coming out, where do you see the cabozantinib/nivolumab combination [falling]? Would you think about that not being used at all or before or after lenvatinib/pembrolizumab?
GILLANI: I think cabozantinib is a great drug as a single agent. It does have activity. If you choose to start with the IO/IO combination, you still have cabozantinib as a second-line option available. Then as third line, you can use the combination lenvatinib/everolimus. I think it’s a very important tool. You just have to play with the dosage and make sure the patient is able to tolerate it. Frequently, people are not able to tolerate the full 60-mg dose and you have to dose reduce them to 40 mg or even 20 mg or 20 alternating with 40 mg.
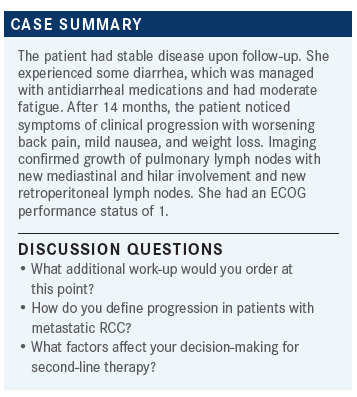
LEE: What additional work-up, if any, would you do at this point? Or would you just change systemic therapy?
DESHPANDE: I might send a tumor profiling. I haven’t seen a lot of data on changing treatment based on that. If something unusual came up, like an NTRK fusion, it might improve the options.
REZNIKOFF: The diarrhea worries me. I wonder what’s causing that—TKI, IO, or something else. That might affect my therapy.
LEE: It’s an incredibly challenging thing. I think that we’ve always had a very tough time trying to do drug attribution. We’ve gone as far as sending people for sigmoidoscopies with biopsies to try to figure out which direction it’s going if it’s not steroid responsive or poorly steroid responsive and we’re thinking about doing…a tumor necrosis factor inhibitor. At that point, we probably want to prove that the diarrhea is IO related as opposed to something else. I think that with a longer half-life, it certainly is quite challenging to sort that out.
From a second-line therapy standpoint, what are people’s thoughts about what they would be picking for this person?
ILYAS: I would go with different TKI regimen here, rather than using another immunotherapy. That could be either lenvatinib plus everolimus combination or any other unused single-agent TKI. That would be my choice.
KIM: Lenvatinib/pembrolizumab beyond progression after IO…is an option for patients who progress on the IO. I don’t know how to [sequence those], whether I should reserve it for the second line. Because after progression with your IO, then you have many options.
GILLANI: I have a question about response assessment on IO/TKI combination. How much of RECIST criteria do we really use? Do you have to be convinced that there is true progression, is radiographic progression enough, or do you have to have clinical progression plus radiographic progression before you [initiate a change in therapy]?
LEE: On a clinical trial, you just need radiographic progression. For progression-free survival [PFS] cutoff, progression is measured by radiographic progression, [which] would be defined as a greater than 20% increase in the target lesions plus a minimum of 5 mm’s worth of change. Let’s say your target lesion went down to 3 mm and it went up by 1; that’s 33%. But that doesn’t give you the 5-mm minimum change.
The other part of RECIST criteria that comes in is that progression at a nontarget lesion has to be clear progression at a nontarget lesion or the development of a nontarget lesion. It’s counting from nadir. A lot of the trials do allow for treatment beyond progression if we decide that there’s clinical benefit from it. But it’s not something that gets captured in that PFS curve, so that PFS curve was either clinical progression or radiographic progression. Either would be fine.
ILYAS: Were there any times that, in your practice, when deciding between a true progression and pseudoprogression from IO, you chose to ride out that progression, continue with the same regimen, and repeat another short-term imaging? How do you approach that?
LEE: I think that we certainly do see pseudoprogression. There are very differing opinions about the degree of it that we see. For TKI/IO regimen, we very rarely see pseudoprogression. We sometimes see it with ipilimumab/nivolumab. I would say that for pseudoprogression on ipilimumab/nivolumab, it’s a little bit harder and more challenging to clarify. If safe, we often will do the confirmatory scan, if they’re asymptomatic. I think that asymptomatic or improving clinical status is key for us to feel comfortable that this might be pseudoprogression as opposed to clearly progressive disease, because the frequency in which this occurs really is relatively small.
My usual approach with patients is to say that I’ve definitely seen it; it happens frequently enough that I know that it exists as an entity. But it’s not common enough that we should count on it, especially if [they’re feeling well].
REFERENCE
Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
