Pushing Ahead With New Treatments for Nonmetastatic Castration-Resistant Prostate Cancer
Patients with the localized form of CRPC still have no options that are proven to extend overall survival, but preliminary results from the PROSPER trial of enzalutamide and the SPARTAN trial of apalutamide offer them unprecedented hope.
Julie N. Graff, MD
It took many years for investigators to find an effective treatment that could extend survival beyond androgen deprivation therapy (ADT) for patients with metastatic castration-resistant prostate cancer (CRPC). The answer was found in the chemotherapy docetaxel (Taxotere) in 2004. The next 13 years saw the approval of 8 treatments for the same disease: the chemotherapies cabazitaxel (Jevtana) and mitoxantrone (Novantrone), the immunotherapy sipuleucel-T (Provenge), the anti-androgen abiraterone acetate (Zytiga), the androgen receptor blocker enzalutamide (Xtandi), external-beam radiation, radium-223 (Xofigo), and the RANKL inhibitor denosumab (Prolia and Xgeva).1
The same period, however, saw no successful trials against nonmetastatic CRPC, so the number of proven treatments remained, at least in the opening months of this year, where it had begun in 2004: at zero.
Patients with the localized form of CRPC (FIGURE) still have no options that are proven to extend overall survival (OS), but preliminary results from the PROSPER trial of enzalutamide and the SPARTAN trial of apalutamide (Erleada) offer them unprecedented hope. Both drugs, used in conjunction with androgen deprivation therapy (ADT), have demonstrated the ability to delay the metastasis that ultimately kill patients with prostate cancer by an average of about 24 months.
These 2 trials may be remembered as the first in a long line of breakthroughs against nonmetastatic CRPC. The FDA approved both apalutamide and enzalutamide for patients with nonmetastatis CRPC in February and July 2018, respectively. Other experimental compounds are currently being tested as monotherapies against the condition, and both enzalutamide and apalutamide are being tested as part of experimental combinations. Nothing is guaranteed just yet, however, not even the wisdom of using enzalutamide or apalutamide against nonmetastatic CRPC.
Neal D. Shore, MD
“Although it’s clear that there is [an] unprecedented delay in the development of metastasis with the use of enzalutamide or apalutamide in patients with nonmetastatic castration-resistant disease, we can’t be certain that we are prolonging life until we see the final overall survival results from PROSPER and SPARTAN,” said Maha H. Hussain, MD, the lead investigator of the PROSPER trial, in an interview withTargeted Therapies in Oncology.
“We do know, though, that metastases precede diseaserelated death in the majority of patients, so the delay of metastases, which is the terminal phase of the disease, by a median time of 2 years is truly a landmark achievement against this disease. We also know that both drugs produce significant improvements in a range of secondary outcomes, which further supports their clinical benefit,” said Hussain, who is the deputy director and genitourinary oncology program leader at Northwestern University’s Robert H. Lurie Comprehensive Cancer Center. “All those considerations have led the FDA to approve both drugs for nonmetastatic castration-resistant prostate cancer and professional organizations such as the American Urological Association to update their treatment guidelines, even in the absence of overall survival data.”
History of Prostate Cancer Advances
Charles Huggins, MD, and Clarence Hodges, MD, outlined the first effective treatment for systemic prostate cancerwhich was also the first effective treatment for any systemic cancer—in 1941, when they published a seminal paper on patients with prostate cancer treated via either physical castration or chemical castration with estrogen therapy.2A follow-up paper from Huggins et al in the same year confirmed the dramatic benefits of testosterone deprivation in a larger patient population.3
Huggins knew when he published that first paper that some patients developed castration-resistant disease, but it took 63 years to find a viable treatment that extended survival once patients progressed on ADT. Huggins actually discovered, and later research confirmed, that removal of the adrenal gland, which produces low levels of androgens, can improve outcomes in castration-resistant cancers, but the complexity of the procedure and paucity of the benefit prevented widespread adoption.4
Even before the 2004 trials with docetaxel in patients with metastatic CRPC, chemotherapy was known to reduce levels of prostate-specific antigen (PSA) and to reduce cancer-related pain in some patients with hormone-refractory prostate cancer, but concerns about toxicities prevented its use in such patients, who received ADT monotherapy instead.4The 2004 trials showed not only that docetaxel extended life in patients with castration-resistant disease but also that the reduction in cancer burden outweighed the chemotherapy toxicity. That said, the survival benefits were modest. Men receiving the most effective regimen of docetaxel every 3 weeks and prednisone survived a median of 18.9 months, which was just 2.4 months more than the median with the comparator regimen of mitoxantrone and prednisone.5A 2010 trial showed a similarly robust-but-modest survival benefit for cabazitaxel and prednisone in patients who failed on docetaxel, the median survival was 15.1 months for the combination.6
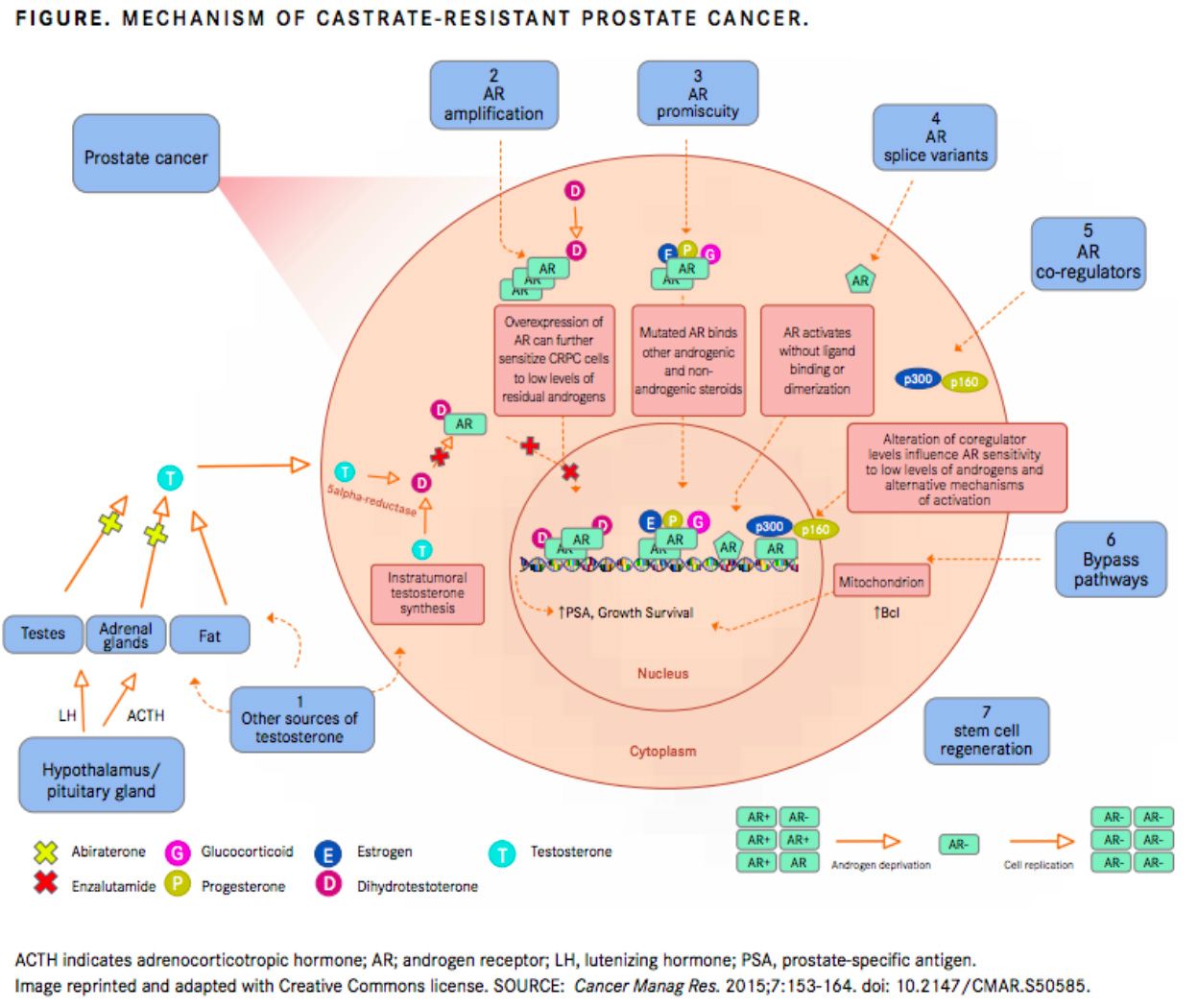
The years that followed saw half a dozen agents approved for metastatic CRPC, but few of those were tested in the setting of nonmetastatic CRPC. Radium-223 and external beam radiation all treat cancer in the bone, so there was little push to test them in patients whose cancer had not spread to the bone. Observers can only guess why the others were never tested in nonmetastatic CRPC.
Whatever the reason, it certainly wasn’t lack of need. One patient-flow model has suggested that the prevalence of nonmetastatic CRPC in the United States amounted to about 40,000 cases in 2013 and will increase over the next 10 years.7
Antiandrogens Swoop
In Enzalutamide and apalutamide seemed likely to help such patients because they both block the androgen receptors that somehow develop castration resistance and resume their ability to drive tumor growth, despite the lack of androgens in patients who continue on ADT. Both drugs also had fewer adverse effects (AEs) than chemotherapeutics. PROSPER and SPARTAN both started in October 2013, but they were not the first trials of anti-androgens against nonmetastatic CRPC.
First-generation nonsteroidal anti-androgens, such as bicalutamide (Casodex), showed some activity against the disease in early stage trials and have long been common treatments for the condition.8
PROSPER and SPARTAN, which both tested second-generation nonsteroidal anti-androgens, have reported better results to date (TABLE).
The double-blind phase III PROSPER trial randomized 1401 men with nonmetastatic CRPC and a PSA doubling time of 10 months or less 2:1 to either enzalutamide and ADT or placebo and ADT. The primary endpoint was metastasis-free survival (MFS), defined as the time from randomization until death or radiographic progression. Secondary endpoints included PSA response rate, time to PSA progression, and OS.9
As of June 28, 2017, 219 of 933 patients (23%) treated with enzalutamide and 228 of 468 patients (49%) treated with placebo had either developed metastasis or died. The median MFS was 36.6 months in the enzalutamide group versus 14.7 months in the placebo group (HR for metastasis or death, 0.29; 95% CI, 0.24-0.35;P<.001). Nearly half of all patients on placebo but just 15% of all enzalutamide patients had moved on to subsequent antineoplastic therapy by data cutoff, and the median time to such therapy was 39.6 months for patients treated with enzalutamide/ADT and 17.7 months for patients in the ADT-alone arm (P<.001). PSA progression occurred in 22% of patients in the enzalutamide group versus 69% with placebo. The median time to PSA progression was 37.2 months in patients treated with enzalutamide and 3.9 months in those treated with ADT alone (P<.001).
Although patients in the placebo arm were far more likely to progress than those treated with enzalutamide, they were not significantly more likely to die as of the data cutoff date. At the first interim OS analysis, 103 patients (11%) who were treated with enzalutamide and 62 (13%) with placebo had died.
These results have so far been similar to those from the double-blind phase III SPARTAN trial.
Investigators randomized 1207 men with nonmetastatic CRPC and PSA doubling times of 10 months or less in a 2:1 ratio to ADT and either apalutamide or placebo. The primary endpoint was MFS, and secondary endpoints included progression-free survival, OS, and time to symptomatic progression, among others.10
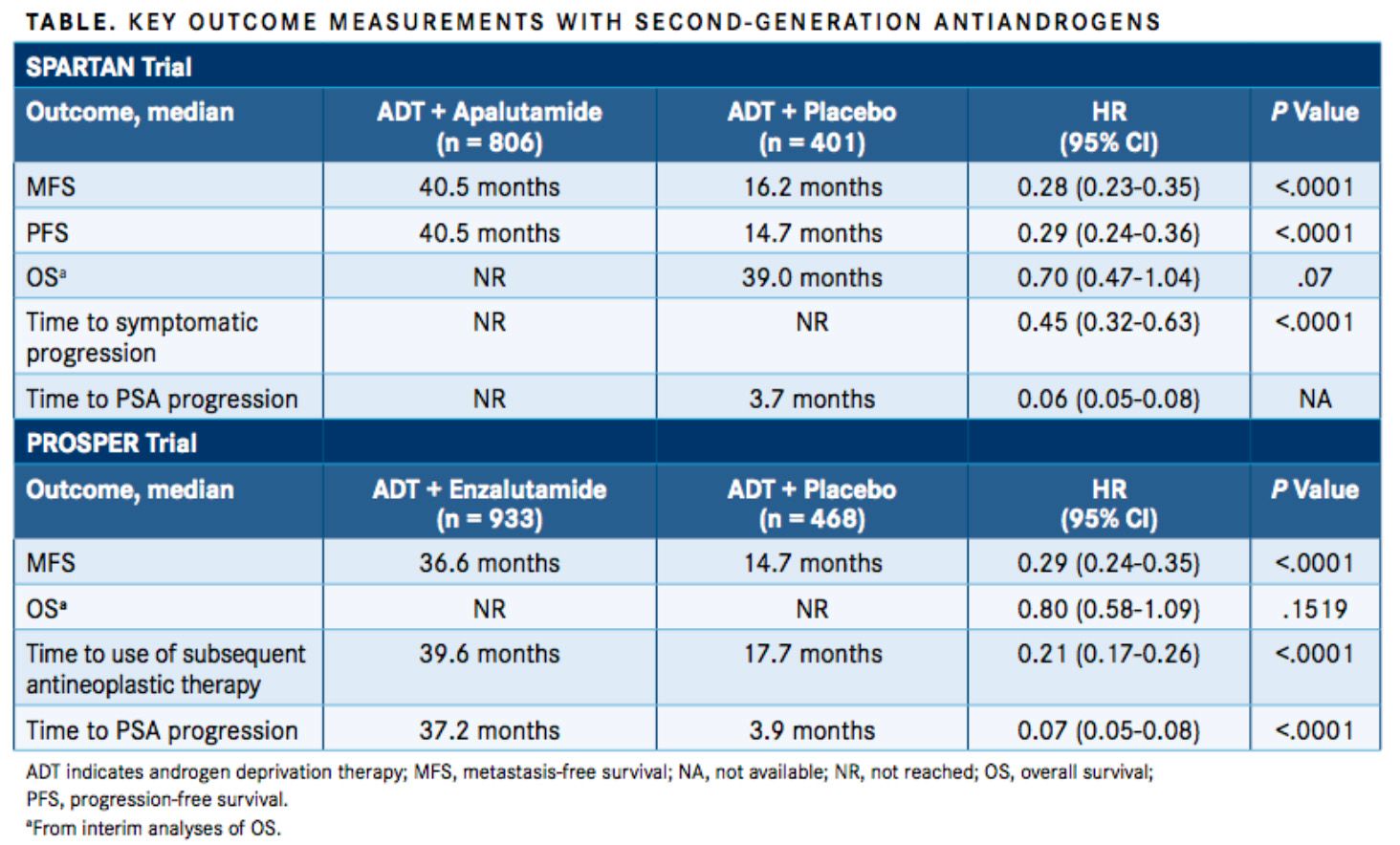
Current published data come from a planned primary analysis that occurred after 378 instances of metastasis or death. The median MFS was 40.5 months in the apalutamide group and 16.2 months in the placebo group (HR for metastasis or death, 0.28; 95% CI, 0.23-0.35;P<.001). Numbers were almost identical for median time to metastasis and median progression-free survival because most of the events were from the development of metastatic disease, not death. The median time to PSA progression was 3.7 months in the placebo group but it was not reached in the apalutamide group (HR, 0.06; 95% CI, 0.05-0.08).
The preliminary analysis did not show a significant increase in OS, which was a median of 39 months among patients receiving placebo and was not yet reached among patients receiving apalutamide, but the calculation was close (HR for death, 0.70; 95% CI, 0.47-1.04,P= .07).
The preliminary SPARTAN data were enough for the independent data and safety monitoring committee to unanimously recommended more than a year ago that the trial be unblinded and that the patients in the placebo group be given the option to receive apalutamide.
Subsequent sub-analyses from the trials have been presented at conferences, and the news has been favorable.
For example, several SPARTAN investigators combed their data to see what they could learn about how well MFS predicted OS. They found that patients who developed metastases at 6, 9, and 12 months died significantly earlier than patients without metastasis, even after adjustment for baseline covariants (Spearman’s correlation coefficient, 0.62;P<.0001). Fleischer’s statistical model confirmed the positive correlation (correlation coefficient, 0.69), with approximately 50% of variability in OS explained by MFS.11
Another SPARTAN sub-analysis combined trial data with existing estimates of patients who would progress from high-risk nonmetastatic CRPC to metastatic disease and then death between 2018 and 2024. They extrapolated from study data to estimate that if all such patients used apalutamide, 92,553 deaths would be averted in those 7 years and 88,682 quality-adjusted life-years would be gained.12
Of course, both of those analyses are necessarily speculative. It would probably take several large trials that improve both MFS and OS in highly correlated ways to know that the first is a good proxy for the second and to infer deaths prevented from improvements in MFS.
What to Do With These Data?
There’s also the matter of weighing the benefits against the financial and physical costs. The wholesale cost of each drug is about $130,00 per year.13,14As for AEs, the SPARTAN trial showed several any-grade AEs that were more common with apalutamide than placebo: fatigue (30.4% vs 21.1%), rash (23.8% vs 5.5%), weight loss (16.1% vs 6.3%) hypothyroidism (8.1% vs 2.0%), and fracture (11.7% vs 6.5%).10Enzalutamide has a similar AE profile, with additional more frequent incidences of hypertension over the placebo group (12% vs 5%).9Of note, both agents show an incidence of mental-impairment disorder of about 5%.9,10
“Many people will think these results already justify giving 1 of these 2 medications to patients with nonmetastatic castration-resistant prostate cancer, but there’s an argument for taking a more conservative approach,” said Julie N. Graff, MD, an investigator on the SPARTAN trial and associate professor of medicine at OHSU Knight Cancer Institute. “The studies haven’t shown significant benefits yet in overall survival. Until we see a benefit in overall survival, we will not know whether you get the maximum benefit by using these medications on nonmetastatic disease or by waiting for metastases. That’s not to say these medicines shouldn’t be used in patients with nonmetastatic castration-resistant prostate cancer, but we still have a lot to learn in terms of timing and long-term toxicity.”
The Institute for Clinical and Economic Review also advocates a wait-and-see approach. A recent report from the research organization said there aren’t enough data yet to choose the proper therapy for nonmetastatic CRPC.15
Further data from both PROSPER and SPARTAN should answer some important questions, but those 2 trials aren’t the only hope for further clarity on optimal treatment strategies.
The ongoing ARAMIS trial is studying a third second-generation anti-androgen called darolutamide in roughly-1500 men with nonmetastatic CRPC (NCT02200614). Early stage trials suggest that darolutamide penetrates the brain and central nervous system less than enzalutamide or apalutamide and thus may produce fewer AEs. The newer medication also binds with a wider variety of androgen receptors, which has led some to hope that it may work somewhat better.16
Another major trial of darolutamide will help answer lingering questions about the efficacy of combining several anti-androgens against prostate cancer while it is still hormone sensitive. The ARASENS trial is testing the combination of darolutamide, ADT, and docetaxel against placebo, ADT, and docetaxel in about 1300 men with newly diagnosed hormone-sensitive metastatic prostate cancer (NCT02799602).16
There are also dozens of smaller trials going on, too, testing different treatments in many combinations on patients at every point in prostate cancer progression. For example, the National Cancer Institute currently lists 21 ongoing trials that combine apalutamide with different forms of medication, radiation, and surgery, and another 49 trials using enzalutamide.
There’s also increasing interest in using immunotherapies besides sipuleucel-T against CRPC. Investigators made news at this year’s ASCO Annual Meeting by announcing some results from a trial of pembrolizumab (Keytruda) in 258 patients with advanced CRPC who had failed all other treatments. In KEYNOTE-199, approximately 40% survived a year and 11% experienced disease control for at least 6 months.17Not many other trials of immunotherapy against advanced prostate cancer have made as many headlines, but dozens of such studies are currently underway.
“There’s been, and continues to be, true excitement within prostate cancer research,” said Neal D. Shore, MD, medical director for the Carolina Urologic Research Center, who was an investigator on the PROSPER trial. “After many years of failed combination trials for castration-resistant disease, after the approval of docetaxel, we’ve had 7 approved CRPC therapeutics within the last 8 years. Now we have 2 newly approved drugs that have shown the ability, on average, to delay metastases for approximately 2 years.
“These 2 newly approved therapeutics, enzalutamide and apalutamide, may also be well suited for combinations with other medications, radiopharmaceuticals, immunotherapies, or other novel oral antineoplastics, such as PARP inhibitors, EZH2 inhibitors, etc. We continue to strive to accomplish survival prolongation, quality-of-life preservation, and complication prevention for our patients [with advanced cancer].
References:
- Ritch CR, Cookson MS. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. doi: 10.1136/bmj.i4405.
- Huggins CB, Hodges CV. Studies on prostate cancer: 1. The effects of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1(4):293-297. cancerres.aacrjournals.org/content/1/4/293.
- Huggins C, Stephens RC, Hodges CV. Studies on prostatic cancer II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43(2):209-223. doi: 10.1001/archsurg.1941.01210140043004.
- 4. Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2(5):389-396. doi: 10.1038/nrc801.
- Tannock IF, de Wit R, Berry WR, et al; TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512. doi: 10.1056/NEJMoa040720.
- de Bono JS, Oudard S, Ozguroglu M, et al; TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154. doi: 10.1016/S0140-6736(10)61389-X.
- Liede A, Arellano J, Hechmati G, et al. International prevalence of nonmetastatic (M0) castration-resistant prostate cancer (CRPC). J Clin Oncol. 2013;31(suppl 15):e16052. ascopubs.org/doi/abs/10.1200/jco.2013.31.15_suppl.e16052.
- Luo J, Beer TM, Graff JN. Treatment of nonmetastatic castration-resistant prostate cancer. Oncology (Williston Park). 2016;30(4):336-344.
- Hussain M, Fizazi K, Saad F, et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378(26):2465-2474. doi: 10.1056/NEJMoa1800536.
- Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418. doi: 10.1056/NEJMoa1715546.
- Smith MR, Mehra M, Nair S, et al. Association of metastasis-free survival (MFS) and overall survival (OS) in nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2018;36(suppl; abstr 5032). meetinglibrary.asco.org/record/162025/abstract.
- Small EJ, Saad F, Zheng Y, et al. Impact of intervening in high-risk nonmetastatic castration-resistant prostate cancer (HRnmCRPC) on metastatic castration-resistant prostate cancer (mCRPC) disease burden. J Clin Oncol. 2018;36(suppl; abstr e17010). meetinglibrary.asco.org/record/161311/abstract.
- Knowledge Ecology International. Selected drug prices database. docs.google.com/spreadsheets/d/1pNGEybsksMVZ_hZnVKVjS2lrjC2aAnwIyNw5ghSg_us/edit#gid=1327374767. Accessed August 27, 2018.
- Carroll J. J&J wins snap approval of apalutamide, the first drug OK’d for nonmetastatic prostate cancer. Endpoints News. February 15, 2018. endpts.com/jj-wins-snap-approval-of-apalutamide-the-first-drug-approved-for-nonmetastatic-prostate-cancer/. Accessed August 27, 2018.
- Antiandrogen therapies for nonmetastatic castration-resistant prostate cancer: effectiveness and value: evidence report. Institute for Clinical and Economic Review website. icer-review.org/wp-content/uploads/2018/08/ICER_Prostate_Cancer_Evidence_Report_082318.pdf. Published August 24, 2018. Accessed August 27, 2018.
- Smith MR, Saad F, Hussain M, et al. ARASENS: a phase 3 trial of darolutamide in combination with docetaxel for men with metastatic hormone-sensitive prostate cancer (mHSPC). J Clin Oncol. 2018;36(suppl 6; abstr TPS383). doi: 10.1200/JCO.2018.36.6_suppl.TPS383.
- De Bonos JS, Goh JCH, Ojamaa K, et al. KEYNOTE-199: Pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2018;36(suppl; abstr 5007). meetinglibrary.asco.org/record/160540/abstract.
