Patel Weighs Role of PD-L1 Expression in Using Immunotherapy in NSCLC
During a Targeted Oncology™ Case-Based Roundtable™ event, Sandip P. Patel, MD, and other oncologists discussed the role of chemotherapy plus dual immunotherapy in patients with non–small cell lung cancer.
Sandip P. Patel, MD (MODERATOR)
Professor of Medicine
UC San Diego Health
San Diego, CA

EVENT REGION Washington
PARTICIPANTS Rafael Santana-Davila, MD | Zheng Wu, MD, PhD | Sharmila Ahmed, MD | Malcolm Winter, MD | Wayne Gao, MD, PhD | Amanda Sun, MD, PhD | Richard Kosierowski, MD | Vik M. Dabhi, MD, PhD
CASE SUMMARY
A 62-year-old woman presented to the emergency department with vague complaints of voice changes and cough. She had a history of recent weight loss of 11 lb. A CT scan of the head and neck area discovered a 1-cm nodule in the left upper lobe. A brain MRI was negative for brain metastases.
She had a medical history of hypertension, hyperlipidemia, and chronic obstructive pulmonary disease (managed on inhalers). She had a smoking history of 10 to 15 pack-years and stopped 25 years ago. Her mother died from lung cancer, aged 65 years. She weighed 125 lb and had an ECOG performance status of 1. Final pathology led to a diagnosis of metastatic stage IV squamous cell carcinoma with metastasis in the liver but no brain metastases. PD-L1 expression by immunohistochemistry was 0%. Next-generation sequencing showed no actionable mutations. Treatment options were reviewed with the patient and family.
The patient was initiated on nivolumab (Opdivo) 360 mg intravenously (IV) every 3 weeks, ipilimumab (Yervoy) 1 mg/kg IV every 6 weeks, and 2 cycles of chemotherapy every 3 weeks.
DISCUSSION QUESTIONS
- In which patients have you considered using nivolumab/ipilimumab plus 2 cycles of chemotherapy?
- What other regimen would you have used and why?
- What clinical and disease characteristics might influence your decision to use this regimen?
DAVILA: I’ve done [this regimen]. There’s no clear setting in which to do it. I’ve particularly done it with patients who are young, otherwise very fit, have a large volume of disease, and for whom single-agent immunotherapy is not indicated.
PATEL: That’s a great subgroup of patients. Those are a lot of host factors that drive therapeutic decision-making which are very relevant.
WU: I use this regimen if there is PD-L1 expression [less than 1%]. I feel like the dual immunotherapy can provide a better chance of cancer control.
AHMED: I’ve used this in patients who can’t tolerate a lot of chemotherapy…[since] we only have to give 2 cycles of chemotherapy [with this regimen]. I found that it’s well tolerated, and with durable responses. I have 1 patient, for example, who had advanced bulky disease. I treated her 3 years ago, and at her most recent CT scan she had no evidence of active disease, which was wonderful to see in metastatic lung cancer.
WINTER: I agree with what others have said. The other thing is by using the chemotherapy up front, sometimes you get a quicker response than from immunotherapy by itself. You get the best of both and minimize toxicities.
PATEL: It’s generally for younger patients, though for someone who is not the best candidate for chemotherapy, [it could be used in] an older patient, but they would need to be able to tolerate colitis as a potential adverse event [AE]. Otherwise, the 2 cycles of chemotherapy are well tolerated.
I typically think about this regimen in patients who are PD-L1 negative with squamous histology, and in patients with brain metastasis. For [those with] adenocarcinoma, [it could be used in] patients with STK11 and KEAP1 mutations who likely need something [extra] because that’s a very immunologically resistant phenotype.
GAO: I don’t see any data for chemotherapy plus nivolumab vs chemotherapy plus nivolumab plus ipilimumab in patients with PD-L1 composite positive score zero. But I did see some that have shown that if the PD-L1 expression is from 1% to 49%, you could use the combination, and it will probably be better.1
PATEL: The closest we have to comparator data is the CheckMate 227 study [NCT02477826], and it’s not a formal comparison, but chemotherapy plus nivolumab was compared with chemotherapy alone, and was compared with nivolumab/ipilimumab. That’s not exactly the proper comparison. Nivolumab/ipilimumab for patients with negative PD-L1 expression did better than chemotherapy plus nivolumab and did better than chemotherapy alone.2 But you’re absolutely right; there hasn’t been a prospective randomized study that compares chemotherapy/PD-1 inhibitor [with or without ipilimumab]. A lot of this is looking across subgroup analyses for existing studies, not only for ipilimumab but also tremelimumab [Imjudo], which is a CTLA-4 blocker mostly commonly used with durvalumab [Imfinzi].
SUN: I have not used this regimen. I have an older patient [in whom] I’m thinking of using this, but their PD-L1 expression is 0% and their tumor mutational burden [TMB] is 14 mutations/megabase. I don’t know if this is a good choice for a 74-year-old patient with reasonable comorbidities and performance status. I was debating whether I should use the combination of ipilimumab/nivolumab or just the chemotherapy plus pembrolizumab approach.
PATEL: There is no level 1 evidence to decide. The PD-L1 negativity may make you think about an ipilimumab/ nivolumab regimen or even the CheckMate 9LA [NCT03215706] regimen, which I use much more commonly. TMB of 10 is high, but in my mind, the [true] high TMB is 20 or greater. The cutoff has just been too low, and that’s why the studies have been negative. But 14 is a reasonable number, and even if they’re PD-L1 negative, they have a reasonable chance to respond. If they want the most aggressive option, I would potentially consider a CTLA-4 inhibitor, PD-1 inhibitor, and chemotherapy. If they’re very concerned about AEs, [such as] if they have 5 or 6 comorbidities, maybe do chemotherapy/ pembrolizumab or pembrolizumab alone or ipilimumab/ nivolumab without the chemotherapy. Is this squamous or nonsquamous histology?
SUN: Squamous.
PATEL: Then you’re dealing with taxanes; I struggle getting older patients through their taxane therapy. I typically will use a CheckMate 9LA regimen because it’s 2 cycles of therapy and [finished] in terms of the taxane. I find my older patients tolerate pemetrexed better, but the taxane is rough for them.
SUN: In patients with melanoma or renal cell carcinoma [RCC], the toxicity of ipilimumab is significant. I had a patient with hypophysitis, and one with severe colitis, and one with pneumonitis and nephritis. After we stopped the ipilimumab, they were better. My concern is that [older] patients seem to develop those complications that make their treatment challenging.
PATEL: Pneumonitis tends to be more a PD-1–driven AE. It’s [rare] with the CTLA-4 inhibitor. [Secondly], the dose of ipilimumab is different in thoracic oncology than in melanoma, [which is] 3 mg/kg induction, or RCC, [which is] 1 mg/kg induction every 3 weeks.3 In thoracic oncology, the ipilimumab is given at 1 mg/kg, the RCC dosing, but instead of every 3 weeks, it’s [given] every 6 weeks. There is colitis, but it’s a much lower level than what we see with melanoma and RCC because the dose is lower and the frequency is longer.
DISCUSSION QUESTIONS
- What patient characteristics would you consider when choosing among immunotherapy plus chemotherapy regimens?
- How do you treat patients with PD-L1 expression greater than 1% vs less than 1%?
KOSIEROWSKI: I’m intrigued by adding the CTLA-4 inhibitor in patients with PD-L1 expression less than 1%. I never thought of it that way and that’s a fairly simple thing to remember, and I’ll look critically at that in the future. On the brain metastases side, I’m still [uncertain] since brain metastases come in so many different shapes and sizes.
PATEL: In non–small cell lung cancer, we don’t have any prospective data [for brain metastases]. The only prospective data are a small, randomized phase 2 trial in melanoma for CTLA-4.
GAO: Another question is regarding the AE profile and whether we add nivolumab and ipilimumab to the chemotherapy regimen. It looks that the AE profile is pretty similar between the 2 groups [From the Data1]. I was surprised to see that since my expectation is that more medication probably causes more AEs.
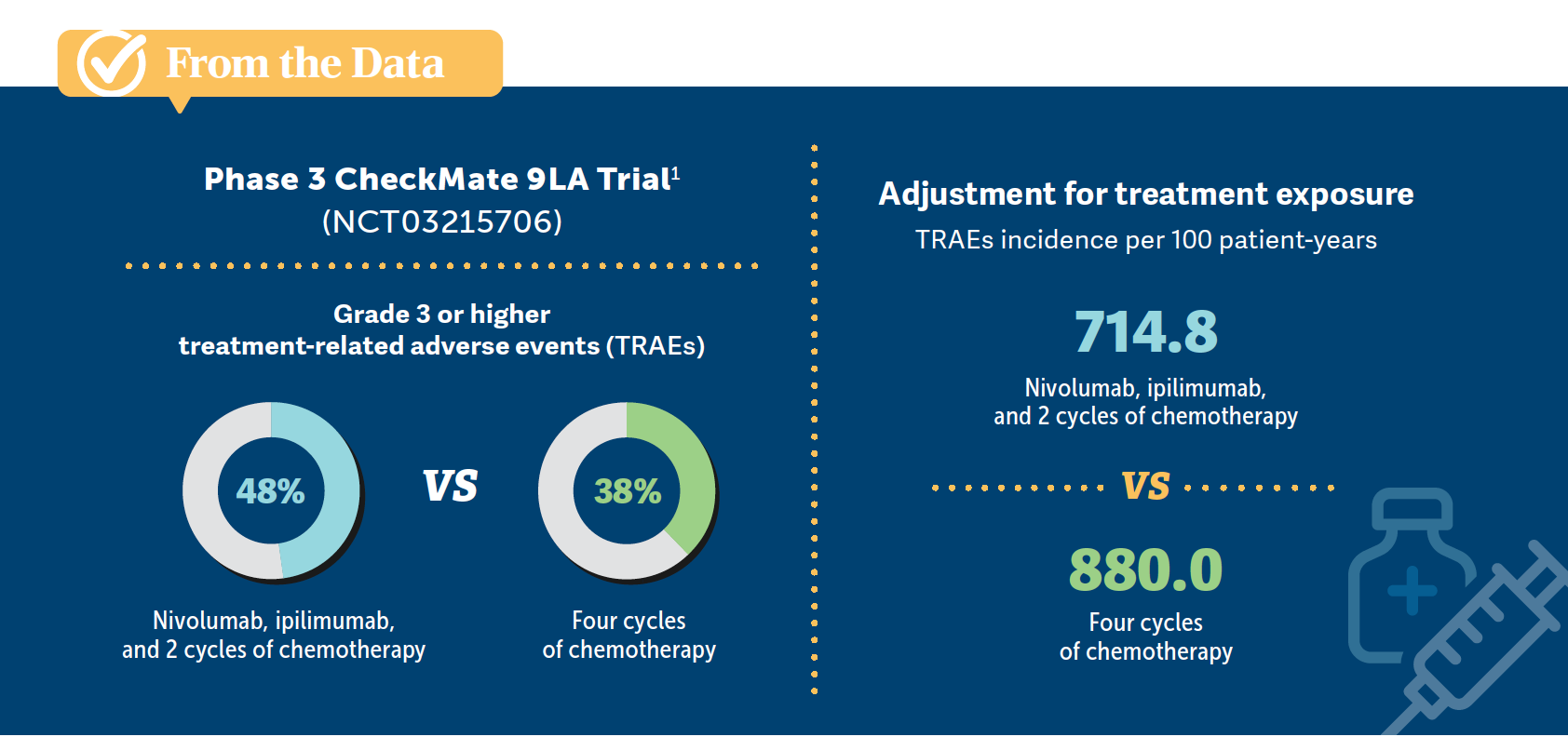
PATEL: The reason for that, at least from the CheckMate 9LA data…is you’re giving less chemotherapy, so you’re getting fewer of the chemotherapy AEs. It’s 2 cycles of chemotherapy [plus immunotherapy] vs 4 cycles for taxane-based [therapy in patients with squamous histology] and 4 cycles plus maintenance for nonsquamous histology. You’re [reducing] the chemotherapy’s [impact on] quality of life, but you’re [adding] low-dose ipilimumab for more immune toxicity. Since it’s not the same high dose as with melanoma or RCC, you’re getting a lower rate of colitis and dermatopathy, and so you’re trading moderate levels of chemotherapy AEs for generally milder immunotherapy AEs. That’s the trade-off that you see in those data.
DABHI: The contribution of CTLA-4 is substantial when added to the PD-L1 inhibitor in the patients with squamous histology with very low PD-L1 expression. Has anyone looked at whether the PD-L1 inhibitor is required in that situation? Since there’s no expression of it, why not just try a CTLA-4 inhibitor with [chemotherapy]?
PATEL: Because the chemotherapy/PD-1 studies were positive in all-comers, or at least the KEYNOTE-021 cohort G study [NCT02039674] was, would it make sense to drop it?4 There was a study [NCT01285609] of chemotherapy plus ipilimumab where the response rates weren’t as high and they were phased vs concurrent approaches.5 It’s a good question, but because KEYNOTE-021 cohort G showed a hint of efficacy in PD-L1–negative disease, and we do see disease that responds, especially with nonsquamous non– small cell lung cancer, the thought is that chemotherapy/ PD-1 is the backbone and there is a niche for CTLA-4 where it may make sense.
DABHI: [There are] some data [that show] patients who have a substantial inflammatory response tend to have a substantial response for the disease and…the treatment is discontinued and the response persists.6 Is there an effort to continue treatment at lower doses or with ongoing suppression using steroids in those situations?
PATEL: It depends on the AE. If they had to discontinue because they had a grade 4 pneumonitis or myocarditis where they’re in the intensive care unit, I would never rechallenge those patients. But [if ] they had a grade 3 colitis, they end up in the hospital but they respond to steroids and fluids, what I’d probably do is continue the PD-1 inhibitor and drop the CTLA-4 inhibitor and drop the chemotherapy.
On the protocols, they were quite strict. Once a patient had a grade 3 AE, they were off the study, and if you thought it was treatment related, you marked that in the electronic data capture. They get immune-related AEs, which we want to be rare, but if they get them, [it means] their immune system is active. Secondly, you don’t need to press so much because the [treatment is already working].
DABHI: I agree with you. You drop one of the drugs. I haven’t seen any commentary on whether one should try lower doses of the PD-L1 inhibitors. There is a bit of information out there about weight-based dosing.
PATEL: I think the weight-based dosing, for most thinner patients, is going to be more cost effective. If you have a heavier patient population, if they’re early in their disease, they have no cachexia yet, the fixed dose probably works out. What you’re trying to do is saturate PD-1 on T cells, which doesn’t correlate 1:1 with weight. It’s not like we’re dosing chemotherapy. Your point is very well taken; there’s not a lot of nuance in how we dose these drugs. It would be much better if we could figure out the dose that would saturate the PD-1 and personalize dosing that way, but the field has moved on to a single infusion of fixed dose where you know you’re saturating all the receptors in a wide swath of patients across the weight spectrum.
DABHI: Fair enough. Then there are no commentaries on patients in whom you might want to just cut the dose down because you’re trying to manage the overstimulation of the immune system?
PATEL: For immune-related AEs…dose reductions tend not to help. You have to hold the drug, and you usually have to give higher-dose steroids, [at] 1 mg/kg. We’ll do the 3- to 4-week steroid taper and then either drop the CTLA-4 inhibitor, drop the chemotherapy, drop the CTLA-4 and PD-1 [inhibitors] and continue the chemotherapy, or maybe just observe depending on what their AE was and where they are in their treatment course.
REFERENCES:
1. Reck M, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5):100273. doi:10.1016/j. esmoop.2021.100273
2. Brahmer JR, Lee JS, Ciuleanu TE, et al. Five-year survival outcomes with nivolumab plus ipilimumab versus chemotherapy as first-line treatment for metastatic non-small-cell lung cancer in CheckMate 227. J Clin Oncol. 2023;41(6):1200-1212. doi:10.1200/JCO.22.01503
3. Yervoy. Prescribing information. Bristol-Myers Squibb; 2023. Accessed January 16, 2024. http://tinyurl.com/yxymszcm
4. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi:10.1016/ S1470-2045(16)30498-3
5. Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449-3457. doi:10.1200/ JCO.2016.71.7629
6. Carbone DP, Ciuleanu TE, Schenker M, et al. First-line (1L) nivolumab (N) + ipilimumab (I) + chemotherapy (C) vs C alone in patients (pts) with metastatic NSCLC (mNSCLC) from CheckMate 9LA: 4-y clinical update and outcomes by tumor histologic subtype (THS). J Clin Oncol. 2023;41(suppl 17):LBA9023. doi:10.1200/JCO.2023.41.17_suppl.LBA9023

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More