Optimal Adjuvant and Neoadjuvant Regimens Are Explored in Lung Cancer
The most effective treatment strategy—adjuvant, neoadjuvant, or some combination of adjuvant plus neoadjuvant regimens— remains unclear. Ongoing trials are dedicated to defining the best sequence of treatment.
Immunotherapy remains breakthrough in the treatment of cancer in recent years, and the emergence of immune checkpoint inhibitors (ICIs) such as PD-L1/PD-1 and CTLA-4, especially in lung cancer, has resulted in improved outcomes in patients. Historically, the ICIs atezolizumab (Tecentriq), durvalumab (Imfinzi), ipilimumab (Yervoy), nivolumab (Opdivo), and pembrolizumab (Keytruda) have gained standard of care status in early and metastatic stages of the disease. Efforts to reach optimal benefit focus on the use of immune biomarkers prior to ICI therapy; unique combinations of ICIs and chemotherapy, radiotherapy, and tyrosine kinase inhibitors (TKIs); sequencing of ICI agents, treatment of duration of ICI therapy; and use of ICI agents stratified by patients’ histopathology, stage, and performance status.
Notable data sets of HER2-mutant disease, as well as targeted agents in the neoadjuvant and adjuvant settings, were explored during 2021.
For example, the DESTINY-Lung01 (NCT03505710) trial assessed the efficacy and safety of trastuzumab deruxtecan (T-DXd), a HER2 antibody-drug conjugate (ADC), in patients with HER2-mutated non–small cell lung cancer (NSCLC). Findings were announced during the European Society of Medical Oncology Congress 2021 and simultaneously published in the New England Journal of Medicine.1,2 In this study, 91 patients were enrolled and followed for a median of 13.1 months (range, 0.7-29.1).
An objective response rate (ORR) of 55% was reported in patients (95% CI, 44%-65%) and the median duration of response was 9.3 months (95% CI, 5.7-14.7). Median progression-free survival (PFS) and median overall survival (OS) were reported as 8.2 months and 17.8 months, respectively. The agent’s safety profile was similar to previously reported studies, with 46% of patients reporting grade 3 or higher treatment-related adverse events (TRAEs). The most common TRAE reported was neutropenia, in 19% of patients.
Investigators reported that adjudicated drug-related interstitial lung disease (ILD) occurred in 24 patients (3 were grade 1, 15 were grade 2, 4 were grade 3, and 2 were grade 5). Additional biomarker analyses showed responses across different HER2-mutated subtypes, as well as in patients with no detectable HER2 expression or HER2 gene amplification.
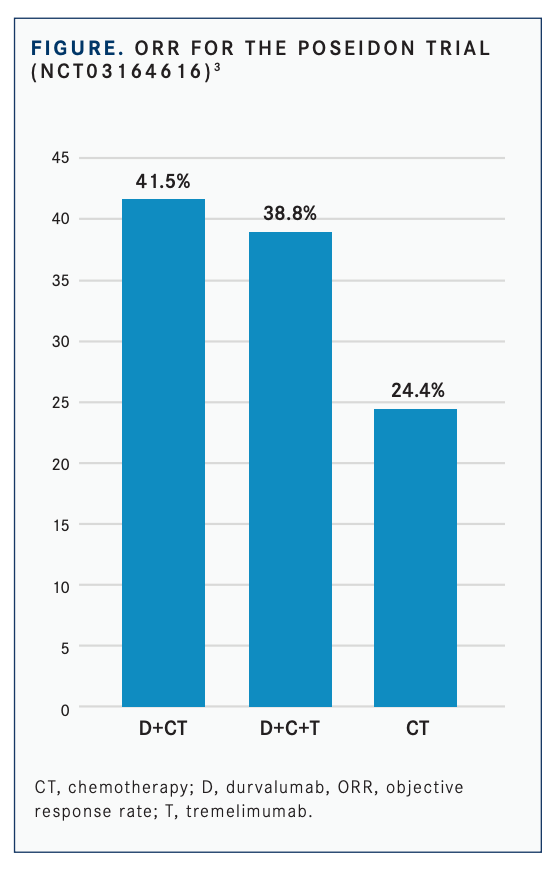
Additional trials refined immunotherapy combinations that helped clinicians identify optimal regimens. The phase 3 POSEIDON trial (NCT03164616) showed that durvalumab and chemotherapy, with or without tremelimumab, led to a statistically significant improvement in PFS compared with chemotherapy alone in patients with NSCLC.3
The findings, presented during the International Association for the Study of Lung Cancer’s 2021 World Conference on Lung Cancer, showed that the median PFS with durvalumab/chemotherapy was 5.5 months (95% CI, 4.7-6.5) compared with 4.8 months (95% CI, 4.6-5.8) for chemotherapy alone, leading to a 26% reduction in the risk of disease progression or death (HR, 0.74; 95% CI, 0.62-0.89; P = .00093).
The median OS in the combination arm was 13.3 months (95% CI, 11.4-14.7) compared with 11.7 months (95% CI, 10.5-13.1) for the solo arm, a difference that was not statistically signifi cant (HR, 0.86; 95% CI, 0.72-1.02; P = .07581).
When tremelimumab was added to the combination therapy, investigators reported an improvement in PFS and OS. In that instance, the median PFS was 6.2 months (95% CI, 5.0-6.5) vs 4.8 months (95% CI, 4.6-5.8) with chemotherapy alone (HR, 0.72; 95% CI, 0.60-0.86; P = .00031). The median OS was 14.0 months (95% CI, 11.7-16.1) and 11.7 months (95% CI, 10.5-13.1) in the 3-drug and chemotherapy-alone arms, respectively (HR, 0.77; 95% CI, 0.65- 0.92; P = .00304).
For POSEIDON, primary end points were PFS by blinded independent central review (BICR) and OS for durvalumab/chemotherapy vs chemotherapy alone. Secondary end points were PFS by BICR and OS, as well as OS in patients with blood tumor mutational burden (≥ 20 mutations per megabase) for durvalumab/tremelimumab plus chemotherapy vs chemotherapy alone.
Additional outcome measures included ORR, duration of response (DOR), and best objective response by BICR, as well as 1-year PFS rate, health-related quality of life, safety, and tolerability.
The confirmed ORRs were 41.5% with durvalumab/chemotherapy, 38.8% with durvalumab/tremelimumab plus chemotherapy, and 24.4% with chemotherapy alone (FIGURE3 ). The odds ratios were 2.26 (95% CI, 1.61-3.19) between durvalumab/chemotherapy and chemotherapy alone and 2.00 (95% CI, 1.43- 2.81) between durvalumab/tremelimumab plus chemotherapy and chemotherapy alone.
Neoadjuvant Setting
There have been a number of targeted therapies explored in the neoadjuvant setting. Updated findings from a phase 2 trial (NCT03433469) that evaluated neoadjuvant osimertinib (Tagrisso), a third-generation EGFR TKI, showed an induced pathologic complete response (pCR) rate of 69% in patients with resectable EGFR-mutant NSCLC.4
In the study, patients receive osimertinib 80 mg orally once daily for 28 days for a minimum of 1 cycle prior to surgical resection or until disease progression or unacceptable toxicity. Investigators may give a second cycle of osimertinib prior to surgery if clinically indicated, and patients may receive up to 2 weeks of additional therapy while awaiting surgery.
The primary outcome is major pCR, defined as at least 10% residual viable tumor following neoadjuvant osimertinib. Additional efficacy end points include tumor downstaging, objective response, pCR rate, and 5-year disease-free and overall survival rates.
In a detailed analysis, investigators reported pathologic response for the 13 patients treated with osimertinib. Six patients had L858R substitutions, and 7 had exon 19 deletions (del19).
Among patients with L858R substitutions, a partial response was observed in 1. This patient remained on treatment for 82 days, and the best radiographic response was –50%. The durations of treatment for the remaining 5 patients with stable disease were 64, 37, 62, 62, and 43 days; the corresponding best radiographic response rates for these patients were –29%, –9%, 0%, –25%, and –7%, respectively.
There are no approved neoadjuvant EGFR TKIs for resectable NSCLC. Investigators have assessed various agents in this setting for patients with EGFR-mutant NSCLC, and results have demonstrated trends toward improvements in pathologic response rates. Osimertinib is approved as an adjuvant therapy after tumor resection in patients with NSCLC whose tumors have EGFR exon del19 or exon 21 L858R mutations.5 The drug’s approval was based on findings from the phase 3 ADAURA trial (NCT02511106), which compared the third-generation EGFR TKI with placebo in patients with fully resected stage IB to IIIA EGFR-mutant NSCLC.
In the phase 2 randomized NEOSTAR trial (NCT03158129), investigators evaluated neoadjuvant nivolumab or nivolumab/ ipilimumab.6 The study followed 44 patients with operable NSCLC using major pathologic response (MPR) as the primary end point.
The MPR rate for each treatment arm was tested against historical controls of neoadjuvant chemotherapy. The nivolumab/ ipilimumab arm met the prespecified primary end point threshold of 6 MPRs in 21 patients, achieving a 38% MPR rate (8/21). Investigators reported a 22% MPR rate (5/23) in the nivolumab arm. In 37 patients resected on trial, nivolumab and nivolumab/ipilimumab produced MPR rates of 24% (5/21) and 50% (8/16), respectively. Compared with nivolumab, nivolumab/ipilimumab resulted in higher pathologic complete response rates (10% vs 38%), less viable tumor (median 50% vs 9%), and greater frequencies of effector, tissue-resident memory, and effector memory T cells.
Results from the CheckMate 816 (NCT02998528) trial, a randomized, phase 3, open-label study evaluating nivolumab plus chemotherapy vs chemotherapy as neoadjuvant treatment for resectable NSCLC, signifi cantly increased pCR rates vs chemotherapy in the intent-to-treat population (ITT) (24.0% vs 2.2%; odds ratio 13.94 [99% CI 3.49-55.75]; P < .0001).7
Improvement in pCR with nivolumab plus chemotherapy vs chemotherapy alone was consistent across key subgroups including disease stage, PD-L1, and tumor mutational burden. Nivolumab plus chemotherapy also improved MPR rates vs chemotherapy in the ITT population (36.9% vs 8.9%), as well as ORR (53.6% vs 37.4%) and radiographic down-staging rates (30.7% vs 23.5%), according to investigators.
Investigators reported that the trial met its first primary end point with a statistically significant improvement in pCR in patients treated with the combination versus the monotherapy, per independent review. The safety profile of nivolumab plus chemotherapy was consistent with the known profi le of this combination regimen, and the addition of nivolumab did not decrease the ability to perform surgery.
Adjuvant Setting
The FDA approved atezolizumab for adjuvant treatment following resection and platinum-based chemotherapy in patients with stage II to IIIA NSCLC whose tumors have PD-L1 expression on 1% or more of tumor cells.8 The FDA also approved the Ventana PD-L1 (SP263) assay as a companion diagnostic device to select patients with NSCLC for adjuvant treatment with atezolizumab.
The major efficacy outcome measure was disease-free survival (DFS) as assessed by the investigator in the primary efficacy analysis population (n = 476) of patients with stage II to IIIA NSCLC with PD-L1 expression on 1% or more of tumor cells (TC) (PD-L1 ≥ 1% TC). Median DFS was not reached (95% CI, 36.1-not estimable [NE]) in patients on the atezolizumab arm compared with 35.3 months (95% CI, 29.0-NE) on the best supportive care (BSC) arm (HR 0.66; 95% CI: 0.50, 0.88; P = .004).
In a prespecified secondary subgroup analysis of patients with PD-L1 TC 50% or greater stage II to IIIA NSCLC, the DFS HR was 0.43 (95% CI, 0.27-0.68). In an exploratory subgroup analysis of patients with PD-L1 TC 1% to 49% stage II to IIIA NSCLC, the DFS HR was 0.87 (95% CI, 0.60-1.26).
The most common (≥ 10%) adverse reactions in patients receiving atezolizumab, including laboratory abnormalities, were increased aspartate aminotransferase, increased blood creatinine, increased alanine aminotransferase, hyperkalemia, rash, cough, hypothyroidism, pyrexia, fatigue/ asthenia, musculoskeletal pain, peripheral neuropathy, arthralgia, and pruritus.
During the American Society of Clinical Oncology 2021 Annual Meeting, findings from IMpower010 (NCT02486718), which evaluated atezolizumab, demonstrated a 34% reduction in the risk of disease recurrence or death (HR, 0.66; 95% CI, 0.50-0.88) compared with BSC among patients with resected PD-L1–positive stage II to IIIA NSCLC.9
In this study, investigators sought to expand the benefits of immunotherapy to patients with stage IB to IIIA disease who had undergone complete tumor resection followed by chemotherapy with cisplatin plus pemetrexed (Alimta), gemcitabine, docetaxel, or vinorelbine for 1 to 4 cycles. Following those treatments, 1005 patients were then randomized to receive either atezolizumab at 1200 mg every 21 days for 16 or BSC.
Participants were stratified by PD-L1 expression status at study entry. Primary end points were DFS in patients with stage II to IIIA disease with PD-L1 expression on 1% or more of tumor cells; all randomized patients in the stage II to IIIA cohort; and the ITT population with stage IB to IIIA disease. For all randomized patients with stage II to IIIA disease, the HR was 0.79 (95% CI, 0.64- 0.96; P = .02). In the ITT population, the DFS did not cross the threshold for statistical significance (HR, 0.81; 95% CI, 0.67-0.99; P = .04).
NADIM-ADJUVANT (NCT04564157) is a phase 3 study of adjuvant chemotherapy vs chemoimmunotherapy for patients with stage IB to IIIA completely resected NSCLC.9
The primary objective is DFS, defined as time from randomization to the earliest event defined as disease recurrence, any new lung cancer (even in the opposite lung), or death from any cause at any known point in time.
In this trial launched in January 2021, 210 patients will be randomized 1:1 in the experimental arm (n = 105) and receive adjuvant chemotherapy/immunotherapy and maintenance adjuvant immunotherapy. In the control arm, patients will receive adjuvant chemotherapy (n = 105).
Patients randomized to the experimental arm will receive nivolumab 360 mg plus paclitaxel 200 mg/m2 plus carboplatin as adjuvant treatment followed by maintenance adjuvant treatment for 6 cycles with nivolumab 480 mg every 4 weeks (+/- 3 days). Patients randomized to the control arm will receive paclitaxel 200 mg/m2 plus carboplatin followed by 2 observation visits. The estimated completion date is June 2027.
Overall, neoadjuvant immunotherapy has demonstrated encouraging activity and favorable safety in patients with NSCLC, with early findings of immunotherapy combinations showing promise. The most effective treatment strategy—adjuvant, neoadjuvant, or some combination of adjuvant plus neoadjuvant regimens— remains unclear. Ongoing trials are dedicated to defining the best sequence of treatment.
REFERENCES:
1. Li BT, Smith EFF, Goto Y, et al. Primary data from DESTINY-Lung01: a phase II trial of trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-mutated (HER2m) metastatic non-small cell lung cancer (NSCLC). Ann Oncol. 2021;32(suppl 5):S1283-S1346. doi:10.1016/ annonc/annonc741
2. Li BT, Smith EFF, Goto Y, et al; DESTINY-Lung01 Trial Investigators. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. Published online September 18, 2021. doi:10.1056/ NEJMoa2112431
3. Johnson ML, Cho BC, Luft A, et al. Durvalumab ± tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. Presented at: International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract PL02.01.
4. Blakely C. A phase II trial of neoadjuvant osimertinib for surgically resectable EGFR-mutant non-small cell lung cancer: updated results. Presented at: International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer. September 8-14, 2021; virtual. Abstract P26.02.
5. FDA approves osimertinib as adjuvant therapy for non-small cell lung cancer with EGFR mutations. News release. FDA. December 18, 2020. Accessed September 9, 2021. Bit.ly/3hipZB9
6. Cascone T, William WN Jr, Weissferdt A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27(3):504- 514. Doi:10.1038/s41591-020-01224-2
7. Forde PM, Spicer J, Lu S, et al. Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. Presented at: American Association for Cancer Research Annual Meeting 2021, April 10-15, 2021; May 17-21, 2021; virtual. Abstract CT003. https://bit.ly/3l7d7jp
8. FDA approves atezolizumab as adjuvant treatment for non-small cell lung cancer. News release. FDA. October 15, 2021. Accessed November 29, 2021. bit.ly/3rcMTQd
9. Wakelee HA, Altorki NK, Zhou C, et al. IMpower010: primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol. 2021;39(suppl 15):8500. doi:10.1200/JCO.2021.39.15_suppl.8500
