Oncogene Identification and Treatment Advances Guide Lung Cancer Meeting
With the identification of new oncogenes leading to the development of drugs that target mutations, lung cancer can now be considered a treatable disease. In addition to advances in therapy, improved tumor testing can help the oncologist develop a personalized approach to treating patients.
David R. Gandara, MD

With the identification of new oncogenes leading to the development of drugs that target mutations, lung cancer can now be considered a treatable disease. In addition to advances in therapy, improved tumor testing can help the oncologist develop a personalized approach to treating patients.
“Although lung cancer is genomically and immunologically complex, we want to offer our patients our best therapeutic options,” said David R. Gandara, MD, director of the Thoracic Oncology Program at the University of California (UC) Davis Comprehensive Cancer Center in Sacramento, in an interview with Targeted Therapies in Oncology.
An essential aspect of staying up to date regarding the best available therapeutic options is to hear from experts about the latest clinical data and understand how they canbe translated into daily practice during major medical conferences. To deliver those messages representsa unique opportunity forcontinuing medical education (CME) programs that blend in-person networking with 21st century technologies.
Combining the benefits of face-to-face interactions with the broad reach of a virtual meeting space, the 21st Annual International Lung Cancer Congress® (ILCC), cochaired by Gandara and hosted by Physicians’ Education Resource®, LLC (PER®), will be held July 23 through 25, 2020, in Huntington Beach, California. One of the benefits of having a virtual component of the meeting is the ability to broadent the conference’s reach. “Attendees and faculty who cannot attend because of travel restrictions will still be able to participate,” Gandara said. Enduring materials will be especially valuable because all sessions will be recorded in audio and video format, so that attendees and sponsors will be able to have this information available when the conference concludes.
The educational opportunities delivered during the meeting will encompass classic lecture formats, panel discussions with leading multidisciplinary experts, interactive question-and-answer sessions, new and recent drug approvals discussions, guidelines, and a special focus on the use of liquid biopsy.
“The development of drugs for KRAS G12C mutations will be a particularly important topic addressed during the conference,” Gandara said. “Historically, this mutation has been an undruggable target because of the difficulty in developing a drug to inhibit this mutation. But now there are 5 drugs in development, and we’ll be hearing about them at ILCC.”
Liquid Biopsy
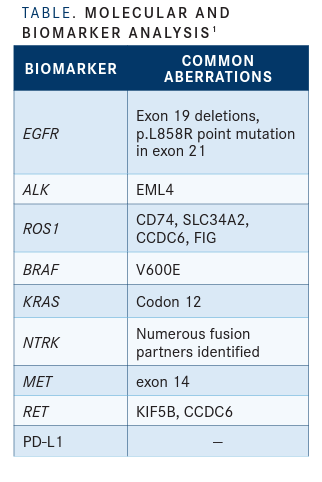
Benefits Currently, the National Comprehensive Cancer Network (NCCN) guidelines for non–small cell lung cancer (NSCLC) recommend testing for 8 oncogene-driven lung cancers before beginning therapy (TABLE).1 The KRAS G12C mutation will be the 9th.
“This means that it’s even more important for practicing oncologists to realize that even a patient who is a previous smoker might have one of these 8 or 9 oncogenes, because KRAS G12C is associated with smoking. The best treatment for them is a targeted therapy, ratherthan chemotherapy or immunotherapy, or even a combination of the two,” Gandara said.
Testing for all these oncogenes in a timely, efficient manner has its challenges, noted Gandara. Genetic panel testing needs to have high sensitivity and specificity, and a have a relatively quick turnaround time of 1 to 2 weeks. “We want oncologists to be able to treat the patient with a personalized or precision medicine approach, rather than an empiric approach,” Gandara said.
Liquid biopsy can help oncologists afford that, providing faster next-generation sequencing that can be carried out simultaneously. This method avoids testing for one gene after another in sequence, which can take a prolonged time. Traditional tissue testing is also limited by the amount of good, viable samples that can be collected during biopsy, and rebiopsy poses a significant challenge to patients.
Molecular Tumor Board
Gandara discussedone particular aspect of the meeting that he’s looking forward to hearing about and that is the Molecular Tumor Board workshop. He explained that the theme of personalized medicine has been well recognized and adopted by clinicians.
In advanced NSCLC, the use of checkpoint immunotherapy in combination with platinum-based chemotherapy is effective; however, among oncologists there has been a divergence of opinion about what this means. For some, this understanding makes treating NSCLC simpler because it suggests that every patient with NSCLC can be treatedwith thisregimen, and Gandara disagrees.
“This regimen makes treating NSCLC more complex because even with the efficacy of the combination of checkpoint immunotherapy and platinum-based chemotherapy,we need to make sure that a patient who comes in doesn’t have one of the oncogenes that will respond even better to a targetedtherapy,” Gandara said.
At ILCC, this debate and a refined definition involving personalized medicine will be addressed during the molecular tumorboard workshop. Casepresentations will be discussed and the audience’s opinion about the best way to treat a particular case will be tallied. “Would you empirically treatthe patient? Or would you wait until the next-generation sequencing results come back?” Gandara asked.
Practice-Changing Developments
Perhaps one of the most practice-changing occurrences in lung cancer recently has been the development of agents that target RET fusions in the disease, as well as the emergence of targeted therapies that have the potential to provide significantly meaningful improvements over the standard of care. RET inhibitors are among the modern strategies used for the treatment of patients with RET fusion–positive cancers, such as NSCLC that is driven by RET fusions.
In the phase I/II LIBRETTO-001 trial (NCT03157128), patients with RET fusion-positive lung cancers and other solid tumors were given selpercatinib. The study assessed themaximumtolerated dose of selpercatinib, recommended phase 2 dose, and objective response rate (ORR) as primary end points. The trial demonstrated an ORR of 68% with selpercatinib in the patientswith RET fusion-positive NSCLC.2
Another important development relates to extensive-stage small cell lung cancer, which has had minor advances in the last 20 years. Standard therapy involved platinum chemotherapy, but in the past year 2 new regimens have beenaddedtothecurrentplatinum–etoposide chemotherapy base, atezolizumab (Tecentriq) and durvalumab (Imfinzi), Gandara said.3,4 Incorporating all these advancements into the workflow of the community oncologist is a key objective of CME activities for conferences such as the ILCC and other major conferences.
Addressing the Elephant
Gandara also shared his experience at the UC Davis Comprehensive Cancer Center delivering care in the face of the coronavirus disease 2019 (COVID-19) pandemic. He said routine follow-up visits have been converted to virtual clinic visits involving telemedicine and videoconferencing. This change also applies to National Cancer Institute (NCI) clinical trials.
“I can see the patient, and they can see me. I can see theirscans and their laboratory results and talk and interact with them,” Gandara said.Importantly, thesetypes of “virtualoffice visits” have been recognized by insurers as reimbursable. Although a physical examination isn’t possible, this methodology provides a way for interval or interim visits to occur without exposing patients to the risk of an office visit and potential exposure to COVID-19.
For those who do need to see a physician, every patient upon entering the UC Davis cancercenterhastheirtemperaturetakenandfills out a questionnaire about symptoms and potential exposure to COVID-19. Patients with a high temperatureor who indicate theyareexperiencingsymptoms associated with this virus are not allowed into the cancer center, but instead are referred to theCOVID-19 referralcenter, where their needs are assessed.
“This is a huge change in practice,” Gandara said.He noted that although many cancer drugs are now orally administered, there remain a select few patients who require infusions." In that case, patients come into the cancer center after they’vebeen checked,and they get their cancer treatment through the infusion center in a safe manner,” Gandara said.
Patients with lung cancer are already a vulnerable population in the age of COVID-19, noted Gandara, as he relayed an interesting anecdote involving social media.He polled practicing oncologists to find out whether they would arbitrarily delay either chemotherapy, immunotherapy,both, or neither in a patient with lung cancer. Of the 357 people who responded, almost 70% said they would treat the patient on schedule, assuming that the patient did not have an active COVID-19 infection.
“A small percentage, about 10%, said they would hold chemotherapy,5% would hold immunotherapy, and about 5% said they would hold both treatment options,”Gandarasaid. “This is open for debate, but my view is that we should treat patients if we can do it safely.” A small number of patients also responded and said that they would like to be treated for their cancer.
Based on the many medical conferences that have been canceled or rescheduled, COVID-19 has an impact on the dissemination of education materials and clinical research results. With the ability to be reimbursed for a virtual office visit, Gandara sees this hurdle being overcome. Further, now that medical meetings can be run on virtual platforms like WebEx or Zoom, Gandara anticipates that CME efforts will change to incorporate more virtual meeting opportunities, but still include the face-to-face component.
“It seems that medicine was heading toward this direction in the first place, but COVID-19 accelerated the pace toward virtual medicine,” Gandara said. “We have used regularly-scheduled WebEx CME programs, grand rounds, and a variety of other platforms and there is good uptake by attendees,” he said.
Achievements
Among Gandara’s many achievements is his continuing role as an educator. He has played an integral part in the ILCC, which is now in its 21st year.
“The ILCC has always been one of the highest- rated cancer conferences, in terms of attendee ratings, and that is quite an accomplishment,” Gandara said.
Additionally, he has served as president of theInternational Association forthe Study of Lung Cancer, or IASLC, leading its conference in San Francisco.
Other achievements include his work in drug development, for which he has received grants from the NCI for nearly 25 years for his research in early-stage treatments. He is a founding cochair of the NCI Investigational Drug Steering Committee, of which he is still a member.
In the clinical trials arena, he has been a stalwart leader for the SWOG Lung Committee for 15 years. During this period, his research with SWOG has resulted in more than 100 clinical trials.
Finally, Gandara values the importance ofmentorship.“When I had a mentor early in my career,it was incredibly important to me. I have tried to be a good mentor as well,” Gandara said. “At this point, I have mentored over 50 young oncologists, PhD students, and medical students. Today, I’m a mentor to 5 different people who are submitting their grant applications.”
References:
1. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer (version 3.2020). Accessed April 23, 2020. https://bit.ly/2xNkJ5
2. Drilon A, Oxnard G, Wirth L, et al. Registrational results of LIBRETTO-001: a phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol. 2019;14(suppl 10):S6-S7. doi:10.1016/j.jtho.2019.08.059
3. FDA approves atezolizumab for extensive-stage small cell lung cancer. FDA. Updated March 18, 2019. Accessed April 23, 2020. https://bit.ly/2yz6H71
4. FDA approves durvalumab for extensive-stage small cell lung cancer. FDA. Updated March 30, 2020. Accessed April 23, 2020. https://bit.ly/2VN7oBX
