New Homologous Recombination Deficiency Findings in Pancreatic Cancer Alter Treatment Focus
PARP inhibitors following platinum-based chemotherapy as well as immunotherapy combinations have shown early signs of efficacy in metastatic pancreatic cancer with homologous recombination deficiency, according to data presented at the 2020 Gastrointestinal Cancers Symposium, held January 23 to 25 in San Francisco, California.
Eileen M. O Reilly, MD

Eileen M. O Reilly, MD
PARP inhibitors following platinum-based chemotherapy as well as immunotherapy combinations have shown early signs of efficacy in metastatic pancreatic cancer with homologous recombination deficiency (HRD), according to data presented at the 2020 Gastrointestinal Cancers Symposium, held January 23 to 25 in San Francisco, California. Recent research also presented at the meeting showed that most patients with a germlineBRCAmutation do not have a family history ofBRCA-related malignancies, further supporting the recent recommendation for universal germline testing in the 2020 National Comprehensive Cancer Network (NCCN) guidelines for pancreatic adenocarcinoma.1However, management of patients with early disease progression remains a challenge.
PARP Inhibitors and Germline BRCA Mutations Olaparib
In December 2019, olaparib (Lynparza) became the first PARP inhibitor approved by the FDA as maintenance therapy for patients with deleterious or suspected deleterious germline BRCA-mutated metastatic pancreatic adenocarcinoma who have not had disease progression on at least 16 weeks of first-line platinum-based chemotherapy.2This approval was largely supported by the results of the POLO study, a double-blind, placebo-controlled, multicenter phase III trial that showed an improvement in progression-free survival (PFS) with olaparib and provided important proof of principle that BRCAmutations in pancreatic cancer predict benefit from PARP inhibitors,3according toEileen M. O’Reilly, MD.
O’Reilly, codirector of medical initiatives at the David M. Rubenstein Center for Pancreatic Cancer Research and section head of hepatopancreatic biliary and neuroendocrine cancers at Memorial Sloan Kettering Cancer Center (MSK) in New York, New York, also noted that by providing an intravenous-free option for maintenance therapy, olaparib may help patients maintain a better quality of life compared with chemotherapy. “A person can take tablets as opposed to needing to come into the clinic to receive infusions,” she said. “It’s a nice quality-of-life consideration for patients in many ways. These responses in select patients can be durable, which means a lot of time away from intravenous treatment.”
Characteristics and Outcomes ofBRCA-Mutated Pancreatic Cancer
The encouraging outcomes observed with PARP inhibition and platinum-based chemotherapy underscore the importance of identifying patients with pancreatic cancer harboring aBRCAmutation prior to treatment. A retrospective analysis of an institutional database at MSK evaluated genomic profiling, clinicopathologic characteristics, and outcomes in patients with pancreatic ductal adenocarcinoma with a germline or somatic mutation in theBRCA1or BRCA2gene.4The analysis identified 126 patients from the MSK database between 2011 and 2018. Of these, 78 patients (62%) had a germline mutation in BRCA1or BRCA2. Among all 126 patients, just 43% and 28% had a family or personal history ofBRCA-associated malignancies, respectively.4According to O’Reilly, restriction of germline testing to patients with factors such as personal or family history of cancer, younger age, or ethnicity that is thought to be associated withBRCA-associated malignancies may mean clinicians are missing a large minority of patients with a pathogenic germline alteration, indicating the importance of germline testing for all individuals with pancreatic cancer. The 2020 update of the NCCN guidelines for pancreatic adenocarcinoma recommends that all patients with a diagnosis of pancreatic cancer receive germline testing using comprehensive gene panels for hereditary cancer syndromes.1
“For these patients, [there are] important findings in terms of the treatment and the information that’s pertinent to their family,” O’Reilly said. "That’s a big message that is getting traction now.”
The study authors also reported a relatively long median overall survival (OS; 32.1 months) compared with historical data from unselect-ed patients with pancreatic cancer. Although they noted thatBRCAmutations may indicate favorable disease biology in pancreatic cancer, the authors also suggested that platinum-based chemotherapy and PARP inhibitors may have contributed to the relatively prolonged survival observed in this subset of patients. Of the 66 patients with stage IV cancer, 43 received first-line platinum-based chemotherapy, including, 36 with a partial response (PR) or complete response (CR). Of the total 126 patients, 44 received a PARP inhibitor (in any treatment line); of these, 15 had a PR and 16 had stable disease (SD). In the 43 patients receiving frontline platinum therapy, 35 had a PR, 1 had a CR, and 4 had SD.4
Although the retrospective analysis showed no difference in OS between patients with somatic and germlineBRCAmutations, O’Reilly noted that more data are needed to determine whether patients with somaticBRCAmutations or alterations in other genes involved in homologous DNA repair, such asPALB2orRAD51, can benefit from PARP inhibitors. “We think that the somatic BRCA mutations that are pathogenic offer the same predictive benefit for olaparib,” she said. “We need more data in that space, and we need better understanding of what the frequency of somaticBRCAmutations [is] in pancreas cancer.”
Veliparib During or After Platinum Chemotherapy
PARP inhibition’s success in the maintenance setting prompted the idea of combining platinum-based chemotherapy with a PARP inhibitor to improve tumor response and prolong PFS and OS. A multicenter, open-label, phase II trial evaluated cisplatin plus gemcitabine with ductal adenocarcinoma harboring a germlineBRCAorPALB2mutation. O’Reilly was the lead author on a report of the results published in January, which noted that the activity of both regimens exceeded prespecified efficacy thresholds, but neither PFS (10.1 vs 9.7 months;P= .73) nor OS (15.5 vs 16.4 months;P= .60) was improved by the addition of veliparib compared with cisplatin and gemcitabine alone. Furthermore, adding veliparib led to more than double the number of grade 3 or 4 hematologic toxicities, and O’Reilly noted that the anemia and thrombocytopenia associated with PARP inhibitors often become dose limiting over time, particularly when combined with platinum-based chemotherapy.5
The study results, which were also present-ed at the symposium, showed 2- and 3-year OS rates of 30.6% and 17.8%, respectively.5An exploratory analysis examined a subset of 10 patients from both study arms (with and without veliparib) who continued with next-line therapy after official study treatment completion. These patients received ≥4 months of platinum-based chemotherapy followed by PARP inhibition and had a median OS of 23.4 months.5,6According to O’Reilly, these findings support the outcomes from POLO. The results indicate that PARP inhibitors will likely have a role as maintenance therapy following platinum-based chemotherapy and that the combination of cisplatin and gemcitabine is an effective chemotherapy regimen for patients with pancreatic cancer associated with HRD.
Immunotherapy Combinations: Ipilimumab and Nivolumab
Although immune checkpoint inhibitors (ICIs) have led to promising outcomes in many types of cancer, they have not shown strong efficacy in unselected patients with pancreatic tumors. This was observed in a recent phase II randomized clinical trial, which demonstrated an objective response rate of 3.1% in the arm that received the antiPD-L1/CTLA4 combination of durvalumab (Imfinzi) plus tremelimumab and no responses in the arm that received durvalumab monotherapy.7
However, tumors with mutations in DNA dam-age repair genes, such asBRCAandRAD51, have features that have been associated with response to ICI therapy. One sequencing-based retrospective cohort study examined mutational signatures in resected pancreatic ductal adeno-carcinoma cases. Of several identified subtypes, the double-strand break repair and mismatch repair deficiency subtypes were associated with increased expression of genes implicated in antitumor immunity, including activation of CD8-positive T lymphocytes and overexpression of CTLA4, PD-L1, and IDO1, which corresponded to increases in the frequency of somatic mutations and levels of tumor-specific neoantigens.8
In a similar study, 450 pancreatic adenocarcinomas were analyzed at the molecular level. Compared with the overall set of tumors, those with mutations inBRCA1orBRCA2had an elevated incidence of PD-1positive tumor-infiltrating lymphocytes and PD-L1 overexpression, respectively.9
However, clinical trials of ICI therapy in patients with HRD cancers have shown mixed results.10
Investigators at the University of Miami Sylvester Comprehensive Cancer Center retrospectively evaluated whether patients with relapsed/refractory pancreatic or biliary cancers who harbored pathogenic germline mutations inBRCAorRAD51responded to immune check-point inhibition. Using their institutional review boardapproved database, the investigators identified patients who had received ipilimumab (Yervoy) plus nivolumab (Opdivo) and performed a retrospective chart review of survival outcomes. The analysis showed that 5 of 7 patients with microsatellite-stable,BRCA- orRAD51-mutated pancreaticobiliary tumors received a clinical benefit from ipilimumab plus nivolumab, with 2 CRs, 1 PR, and 2 cases of SD observed.11
Although the number of patients analyzed was small, O’Reilly said, these results suggest that using ICIs in pancreatic cancer should be further explored in selected patients, perhaps as an adjunct to PARP inhibitors or platinum-based chemotherapy in the setting of HRD.
“We do know that patients who have DNA damage repair gene mutations probably are more likely to respond to immunotherapy agents,” she said. “That piece of evidence is drawn from other malignancies, but there’s some work in the pancreas cancer field that speaks to that, so it’s not out of left field in terms of the observation. Looking at this combination [ipilimumab and nivolumab] or perhaps combining [it] with other things, such as a PARP inhibitor or platinum chemotherapy, would be strategies of particular interest in patients with DNA damage repair gene mutations.”
Factors Associated With Early Progression or Death
Early progression or death (within 4 months of starting therapy) is commonly observed in clinical trials of unselected patients with pancreatic cancer, with approximately half of patients progressing or dying during the first 6 months of treatment with FOLFIRINOX (folinic acid [leucovorin], fluorouracil, irinotecan, and oxaliplatin)12 or nab-paclitaxel (Abraxane) plus gemcitabine.13
In the POLO trial (NCT02184195) of patients withBRCA-mutated pancreatic cancer, the authors noted that 43 (21.7%) of the 198 patients with germlineBRCAmutations screened for entry had experienced disease progression during first-line platinum-based chemotherapy prior to randomization.3O’Reilly said that she and her colleagues observed similar early progression in a small number of patients in their study of cisplatin and gemcitabine with or without veliparib, and she stated that understanding how to “get ahead of the disease for these rapidly progressing patients” will be an important step forward in treatment.
However, studies have not decisively established factors associated with early progression during maintenance therapy. Therefore, investigators involved in the POLO trial aimed to identify such factors in patients receiving maintenance therapy with olaparib.
An analysis of 154 patients randomized in the POLO trial showed that 33 of 92 patients in the olaparib maintenance arm and 29 of 62 in the placebo arm were identified as early progressors (defined as disease progression or death within 4 months of randomization). Multivariate logistic regression analysis using a stepwise selection method was employed to search for baseline factors associated with early progression. The results showed that a lower score on the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item module physical functioning domain, when included as a continuous variable, was the only factor associated with significantly increased risk of early progression. The odds ratio for this association was 0.973; according to the authors, this equated to a 25% change in the odds of early progression for a 10-point difference in the baseline physical functioning score. According to O’Reilly, a poor baseline physical functioning score is likely a proxy for aggressive disease biology and extensive disease burden.14“They have challenging [disease] biology, usually large disease burden, [and] often liver dysfunction because of the large disease burden,” she said.
The authors noted some limitations of the analysis, including the small sample size, which limited its power; the possibility that some patients classified as early progressors already had disease progression at the initiation of study treatment; and exclusion of some patients from the multivariate analysis because of missing baseline covariate data. Additionally, some baseline variablesnamely, neutrophil count, lymphocyte count, and neutrophil-to-lymphocyte ratio—were excluded from the multivariate analysis because of high levels of missing data.14
Going Forward
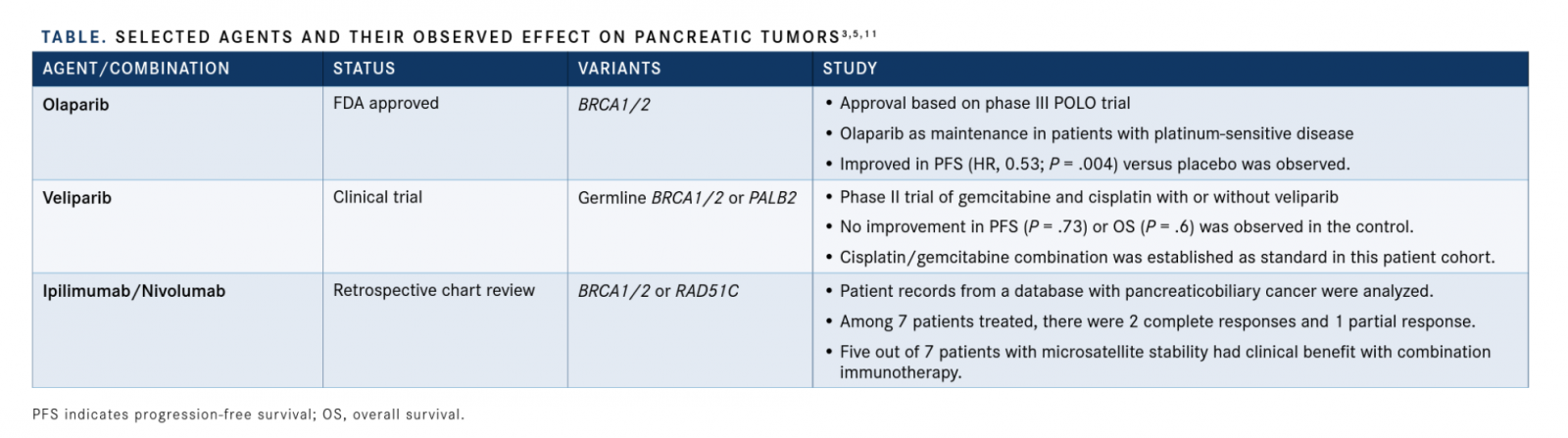
These recent data further support a role for PARP inhibitors in the maintenance setting and introduce checkpoint inhibition as a possible treatment modality for patients with pancreatic cancer harboring a germlineBRCAmutation (TABLE).3-5,11According to O’Reilly, identifying a broader population of patients who may benefit from PARP inhibitors, such as those with somaticBRCAmutations or mutations inPALB2and other homologous repair genes, and finding ways to combine PARP inhibitors with therapies such as ICIs and VEGF inhibitors will be important moving forward. She added that investigations of PARP inhibitors in other settings of pancreatic cancer, such as locally advanced disease and following standard adjuvant therapy in resectable disease, will likely start soon and represent important new directions for the field.
References
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Pancreatic Adenocarcinoma (version 2020). bit.ly/2TI83n8. Accessed February 26, 2020.
- FDA approves olaparib for gBRCAm metastatic pancreatic adenocarcinoma. FDA website. bit.ly/2Ts7jUe. Updated December 30, 2019. Accessed February 26, 2020.
- Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317-327. doi: 10.1056/NEJMoa1903387.
- Momtaz P, O’Connor CA, Chou JF, et al. Pancreatic ductal adenocarcinoma (PDAC), BRCA: analysis and outcomes of cohort from Memorial Sloan Kettering Cancer Center (MSK). J Clin Oncol. 2020;38(suppl 4; abstr 708). doi: 10.1200/JCO.2020.38.4_suppl.708.
- O’Reilly EM, Lee JW, Zalupski M, et al. Randomized, multicenter, phase II trial of gemcitabine and cisplatin with or without veliparib in patients with pancreas adenocarcinoma and a germline BRCA/PALB2 mutation [published online January 24, 2020]. J Clin Oncol. doi: 10.1200/JCO.19.0293
- O’Reilly EM, Lee JW, Zalupski M, et al. A randomized, multicenter, phase II trial of gemcitabine (G), cisplatin (C) +/- veliparib (V) in patients with pancreas adenocarcinoma (PDAC) and a known germline (g)BRCA/ PALB2 mutation. J Clin Oncol. 2020;38(suppl 4; abstr 639). doi: 10.1200/ JCO.2020.38.4_suppl.639.
- O’Reilly EM, Oh DY, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1431-1438. doi: 10.1001/jamaoncol.2019.1588.
- Connor AA, Denroche RE, Jang GH, et al. Association of distinct mutational signatures with correlates of increased immune activity in pancreatic ductal adenocarcinoma.JAMA Oncol. 2017;3(6):774-783. doi: 10.1001/ jamaoncol.2016.3916.
- Millis SZ, Abbott BL, Baker EH, et al. Multiplatform molecular profiling of pancreatic adenocarcinomas to identify BRCA1/2 mutations and PD-1/ PD-L1 status with therapeutic implications. JClin Oncol. 2015;33(suppl 15; abstr 4124). doi: 10.1200/JCO.2015.33.15_suppl.4124.
- Bever KM, Le DT. DNA repair defects and implications for immunotherapy.J Clin Invest. 2018;128(10):4236-4242. doi: 10.1172/JCI122010.
- Terrero G, Pollack T, Sussman DA, et al. Exceptional responses to ipilimumab/nivolumab in patients with advanced pancreaticobiliary cancer and germline BRCA or RAD51 mutations.J Clin Oncol. 2020;38(suppl 4; abstr 754). doi: 10.1200/JCO.2020.38.4_suppl.754.
- Conroy T, Desseigne F, Ychou M, et al; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer.N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923.
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine.N Engl J Med. 2013;369(18):1691-1703. doi: 10.1056/NEJMoa1304369.
- Macarulla T, Kindler HL, Hammel P, et al. Early progression (progr) in patients (pts) with metastatic pancreatic cancer (mPaC) and a germline BRCA mutation (gBRCAm): phase III POLO trial of olaparib (O) versus placebo (P). J Clin Oncol. 2020;38(suppl 4; abstr 750). doi: 10.1200/ JCO.2020.38.4_suppl.750.