Darolutamide Trial Evaluates Noninferiority to Standard Care in Intermediate-Risk Prostate Cancer
External Beam Radiation therapy with or without androgen deprivation therapy or surgery are the standards of care for men with intermediate-risk prostate cancer, but the adverse events associated with the treatments, delayed testosterone recovery and prolonged erectile dysfunction, are challenges.

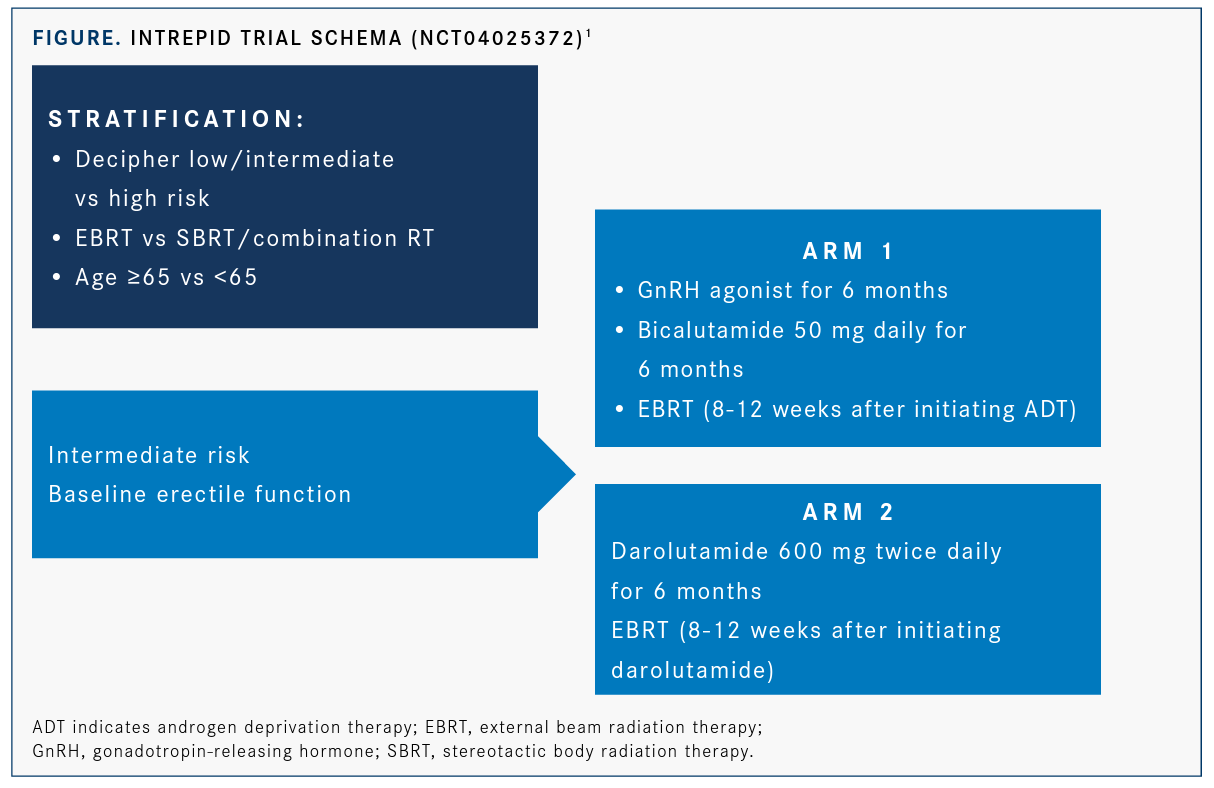
External Beam Radiation therapy (EBRT) with or without androgen deprivation therapy (ADT) or surgery are the standards of care for men with intermediate-risk prostate cancer, but the adverse events associated with the treatments, delayed testosterone recovery and prolonged erectile dysfunction, are challenges. Lead investigator Martin T. King, MD, director, brachytherapy clinical operations at Dana-Farber Cancer Institute and instructor in radiation oncology at Harvard Medical School is seeking to enroll men with intermediate-risk prostate cancer to determine safety, effectiveness and noninferiority to standard care for the hormone therapy darolutamide (Nubeqa) (FIGURE).1Darolutamide is indicated for advanced forms of prostate cancer; however, its activity in intermediate risk prostate cancer is unknown.
“Many of the patients who are diagnosed with prostate cancer are concerned about side effects, which are an important indicator of their quality of life, particularly in the case of erectile dysfunction,” said King, who discussed the INTREPid trial with Targeted Therapies in Oncology, which he presented during the 2020 Genitourinary Cancer Symposium in San Francisco, California, February 13 to 15, 2020.
The phase II INTREPid study will also evaluate erectile dysfunction at baseline, as well as during and after treatment, to determine if short-term erectile dysfunction can be preserved without sacrificing long-term disease control. It is hypothesized that men who receive darolutamide and radiation therapy rather than ADT and radiation therapy are able to achieve surrogate prostate-specific androgen (PSA) end points that are prognostic for long-term disease control while preserving erectile function.
Primary end points of the trial will be the percentage of men with a PSA nadir of ≤0.5, determined 6 months from the completion of treatment. Response is defined as a PSA nadir ≤0.5 within 6 months of the end of treatment. Secondary end points include percentage of patients with good erectile function, PSA progression-free survival, metastasis-free survival, and rate of toxicity.
Eligible patients will have intermediate-risk disease based on National Comprehensive Cancer Network guidelines, will be undergoing radiation therapy with curative intent, good erectile function based on the EPIC-26 Questionnaire, successful acquisition of a Decipher score, and ECOG performance status of 0 to 1. Ineligible patients are those who have had prior surgical, cryotherapy, or high-intensity focused ultrasound for prostate cancer, prior orchiectomy or hormonal therapy, or prior chemotherapy for prostate cancer.
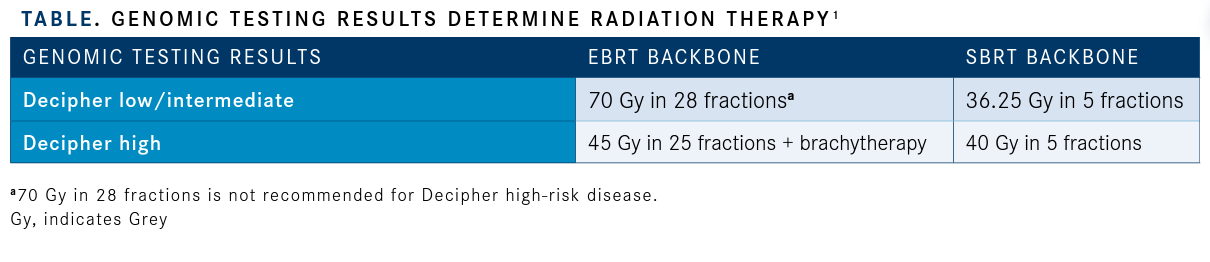
Greater Role for Genomic Testing Genomic testing, delivered by companies such as Decipher, Prolaris, and Oncotype DX, may help stratify high-risk patients beyond traditional criteria, provide guidance in treatment decision making, and determine prognostic outcomes (TABLE).1“In the current trial, investigators will be able to determine if patients with high genomic risk will have a positive reaction to hormonal de-escalation with darolutamide alone or determine if it is only safe for patients with low- or intermediate-risk disease,” King said. “We should be able to get at least some signal on the safety of ADT plus darolutamide in patients with high-risk or low/intermediate- risk disease.”
Another aspect of the trial is that all patients who participate will receive a genomic classification score. The genomic classifier will tell us what the patient’s risk of disease progression could be. Patients with a higher risk score will undergo more extreme radiation dose escalation.
“Hopefully, we will be able to escalate the radiation for men with the most aggressive types of cancers while de-escalating the hormone therapy part,” King said.
King also discussed interesting data that were presented during 2020 Genitourinary Cancer Symposium. He noted that in one study evaluating the quality of life of men with locally advanced or metastatic hormone-sensitive prostate cancer, patients who received abiraterone for the first 2 years of treatment had higher reported quality of life (QOL) scores than patients who received docetaxel for 2 years, especially in the first year of treatment (NCT00268476).2
Patients in the STAMPEDE trial who were randomized to receive either docetaxel or abiraterone completed quality of life questionnaires during treatment and follow up. This sub-study focused on global quality of life the first 2 years after randomization. A total of 173 men received docetaxel and 342 men received abiraterone in this QOL study and contributed to the analysis. At baseline, global QOL scores were similar with the mean in the docetaxel group of 77.8 (standard deviation 20) and abiraterone group of 78.9 (standard deviation of 19.3).
The average global QOL over 2 years was higher in patients randomized to abiraterone than docetaxel, although the difference was statistically significant, it did not meet the predefined clinical parameter of +3.9 (95% CI, 0.6-7.1;P= .021).
Cross-sectional analyses showed clinically meaningful superior QOL in the abiraterone group at 3 and 6 months (+6.6, 95% CI, 2.6-10.7,P= .001; +8.0, 95% CI, 3.6-12.3, P <.001), but not at 1 or 2 years (+1.3, 95% CI, 3.0 to 5.6, P= 0.545; +4.5, 95%CI, 0.25 to 9.2, P= .063).
This finding should be considered when oncologists discuss treatment options with their patients, King said.
References
- King MT, Wise DR, Scala LM, et al. INTREPId (INTermediate Risk Erection PreservatIon Trial): A randomized trial of radiation therapy and darolutamide for prostate cancer.J Clin Oncol. 2020;38(suppl 6; abstr TPS384). doi: 10.1200/JCO.2020.38.6_suppl.TPS384.
- Rush HL, Cook AD, Brawley CD, et al. Comparative quality of life in patients randomized contemporaneously to docetaxel or abiraterone in the STAMPEDE trial.J Clin Oncol. 2020;38 (suppl 6; abstr 14). doi: 10.1200/JCO.2020.38.6_suppl.14.