Morgans Discusses Therapy Options for Metastatic and Nonmetatstatic CRPC
During a Targeted Oncology Case Based Peer Perspectives event, Alicia K. Morgans, MD, MPH, reviewed 2 cases of patients with nonmetastatic castration resistant prostate cancer and explained the potential testing and treatment options.
Alicia K. Morgans, MD, MPH

During a Targeted Oncology Case Based Peer Perspectives event, Alicia K. Morgans, MD, MPH, associate professor of Medicine, Feinberg School of Medicine at Northwestern University in Chicago, IL, reviewed 2 cases of patients with nonmetastatic castration resistant prostate cancer and explained the potential testing and treatment options.
Targeted Oncology: What do you think about hereditary germline testing for this patient?
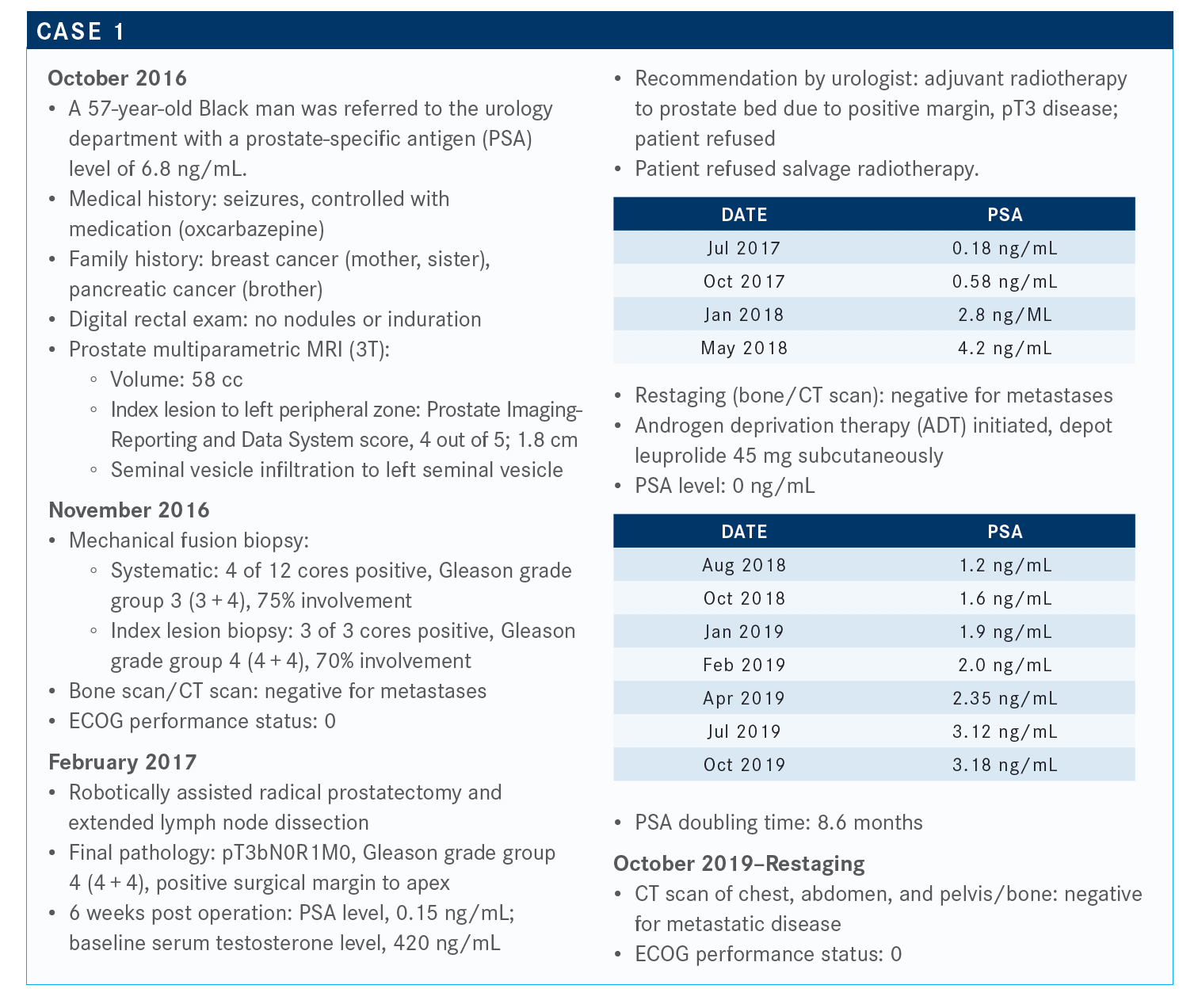
MORGANS: The NCCN [National Comprehensive Cancer Network] has evolved over the last couple of years and, right now, even patients with high-risk localized prostate cancer are recommended to have germline genetic testing. What’s interesting to me, though, is that biochemical recurrence is left out of the guidelines. So, localized [disease] is recommended for testing, metastatic disease recommended for germline genetic testing, but we have this whole biochemical recurrence area that is completely not noticed. That’s probably because we don’t have the data to get it into the guidelines.
What are your thoughts on getting a prostatespecific membrane antigen (PSMA) scan for patients similar to this one?
We’ve had a number of false positives with PSMA; not as many, I think, as with fl uciclovine, although it’s not as sensitive. At the same time, any of these things are reasonable to do. I would be most interested in doing them if the patient was willing to do something [such as] salvage radiation to the prostate bed now that we’re in the castration-resistant state. So it doesn’t make as much sense.
I guess the question is, if you do any of those imaging techniques—which are great, and PSMA will probably be available to us within the next 6 to 12 months—what do you do with the information on the back end? Because other than systemic therapy for nonmetastatic castration-resistant prostate cancer [CRPC]—this patient [has] nonmetastatic CRPC, because that is defined by conventional imaging—we don’t have great algorithms for how to treat these patients. When we do identify something by accident on PSMA PET, we don’t have data to tell us that we can benefit patients either by pelvic radiation or by things [such as] SBRT [stereotactic body radiation therapy] to local spots. There are certain studies that suggest that we could delay systemic therapy, but what does that mean in terms of survival? What does that mean in terms of other end points that are important to us in prostate cancer? I don’t know.
How would PSMA and molecular imaging change your treatment decision-making?
This patient is castration resistant because he’s been on leuprolide for a bit of time at this point. We have several systemic therapies— androgen receptor antagonists—that are approved in this state that we could think about. But it’s important and interesting that all of us think about this new molecular imaging; it’s interesting to think about how that’s going to [change things] in terms of our treatment paradigms, because we don’t have great phase 3 data on treatment options other than systemic therapies in these [patients with] nonmetastatic CRPC that are based on molecular imaging, but it is what’s coming. I don’t know that there’s a perfect answer there.
What are your thoughts on the prognosis for this patient?
This is the kind of patient with a PSA doubling time in the castration-resistant, nonmetastatic state that suggests that he has a high risk of developing metastatic disease or dying from prostate cancer. So, we have a PSA doubling time of less than 10 months, which puts him in a high-risk population.
If you did a PET scan and found low-volume bone lesions, would it qualify the patient for sipuleucel-T (Provenge)?
Yeah, so I think [that depends on] what our defi nitions are. The sipuleucel-T trials were all done using conventional imaging. Patients in the sipuleucel-T study had to have metastatic disease by bone scan, or CT chest, abdomen, pelvis with intravenous contrast. We’re in a situation where this patient does not have any of that. We haven’t done a PET scan yet, so we don’t know if the patient does have a disease, but we are still in the nonmetastatic setting. I’m sure you could ask the insurance company, if they’re willing to pay for that, and [it probably would be paid for]. But you’re not technically [at metastatic disease] yet. Even if we fi nd something on a PET scan, we’re not technically metastatic by the studies that have defi ned the treatments for metastatic CRPC. And that’s the problem. These are shifting sands under our feet. The imaging is not exactly at the same place as the phase 3 trials that led to the approval of all these drugs. So I think that’s a great point. You could probably use sipuleucel-T, but I might even hold that in my back pocket for use after whatever I do next.
How does the patient’s history of seizures affect which treatment you can give him?
In some of the metastatic CRPC studies—enzalutamide [Xtandi] had a low rate of seizure. In all of the nonmetastatic CRPC studies, including PROSPER1 as well as SPARTAN,2 which was the apalutamide [Erleada] nonmetastatic CRPC phase 3 trial, patients who had a seizure history were excluded from those trials. Interestingly, though, in the ARAMIS3 trial…darolutamide [Nubeqa] doesn’t cross the blood-brain barrier, at least as far as we can tell in preclinical models. They included patients who had a seizure history and did not have a high rate of seizures.
Why would you recommend using an androgen receptor (AR)–targeted therapy for this patient?
In the clinical trials that led to the approval of drugs for nonmetastatic CRPC, which at this point have demonstrated not just the prolongation of metastasis-free survival [MFS] but [have also] demonstrated an overall survival [OS] advantage, even the majority of patients in the control arm eventually cross over to treatment with other AR antagonists. You can’t really make up the time. It’s what we always see in prostate cancer: The earlier you start that first treatment, the earlier you can get the benefit.
We’re trying to find a balance between quality of life, getting our treatments in early because we know that that’s important for these patients, and getting something effective into them.

Do you agree with the choice of darolutamide here?
The FDA approved agents for nonmetastatic CRPC [include] apalutamide, enzalutamide, and darolutamide. The history of seizures is something that affected [this decision].
Would your decision be changed if the PSA doubling time were greater?
The FDA label does not require that we have a specific PSA doubling time for any of the AR antagonist agents in this setting. If there are patients that we have certain concerns about, [such as an] issue with a family history or syndrome type picture and [a Black] patient, we certainly are at liberty to use whatever we want. What’s interesting, too, we can even pull these labels in the directions that we want. Sometimes if we find a mess on more sensitive imaging, we can pull the label in that direction. So it’s nice to have that flexibility.
What do the NCCN guidelines recommend for a patient such as this?
We talked about the apalutamide, darolutamide, and enzalutamide, [which have] category 1 recommendations. These are predominantly suggested when the PSA doubling time is less than or equal to 10 months, but the label does not require that to be heard. Sometimes imaging can make these boundaries for indications fluid and can give us the leeway that we want to make other decisions as well.
What data support the use of these guideline-recommended treatments?
The SPARTAN2 trial…was the trial that included apalutamide. These were patients who had nonmetastatic CRPC by conventional imaging again, so it’s a technetium bone scan or a CT [of the chest], abdomen, and pelvis. That was negative for metastatic or radiographic evidence of disease; these patients had to have a PSA doubling time of less than or equal to 10 months in the SPARTAN trial, and particularly, they could have pelvic nodes, but they had to be less than 2 cm in diameter and had to be below the iliac bifurcation. Patients were randomized 2:1 in this trial… to apalutamide versus placebo, both of those given with ADT, and they were followed for a primary end point of MFS, which was novel in the FDA approval world for something that might get through to be approved for the nonmetastatic CRPC setting. They have secondary end points that included OS.
In this study, baseline characteristics were well matched. The median age was around 74, and we could see that a majority of patients had a short PSA doubling time in this study. So 70% or so had a PSA doubling time of less than or equal to 6 months.
This drug was approved because of the prolongation of MFS of about 22 months, apalutamide doing significantly better with ADT than placebo with ADT [40.5 vs 16.2 months]. The hazard ratio [HR] was huge, with an HR of 0.28, [the result is] highly statistically significant [P <.0001], even though a majority of these patients after progression on placebo crossed over to apalutamide. They were not able to make up that survival benefit that we saw.
So, OS data with apalutamide—and this was just released at [the American Society of Clinical Oncology Annual Meeting] this year—demonstrated a median OS of 73.9 months and placebo at 59.9 months, a 22% reduction in mortality [HR, 0.78; P =.0161].4 For this particular treatment, [it was] highly statistically significant, even though there was a large amount of crossover from the placebo arm to the apalutamide arm. The earlier we start, and [we] see this over and over in prostate cancer, the better you do, and you can’t make up that time.
Treatment-related AEs [adverse events] were as we would expect from things [such as] the SPARTAN trial, and we’ve seen this before. There was a fair amount of fatigue and hypertension, and some falls, as compared with placebo. But it was generally well tolerated, and this was consistent in the patient outcomes.
Enzalutamide was in the PROSPER1 trial; this was similar in PSA doubling time of less than or equal to 10 months. We saw conventional imaging that was negative randomized 2:1 to enzalutamide, ADT versus ADT plus placebo, followed again for an MFS end point as the primary end point.
Almost the exact same median age with a median PSA doubling time again, predominantly less than 6 months. [There was] a median PSA doubling time of 3.8 months [with enzalutamide] or 3.6 months [with placebo].
The MFS end point: again 32% or 22 months longer [36.6 months] MFS with enzalutamide and ADT versus [14.7 months with] placebo and ADT [HR, 0.29; P <.0001], and looking at the OS data again, an improvement in OS [67.0 vs 56.3 months; HR, 0.73; P =.001]. There was a 27% reduction in mortality, even though a large proportion of these patients crossed over from placebo to enzalutamide.
[The safety profile was] similar to what we already understand for enzalutamide—some hypertension, some fatigue, some falls. ARAMIS3 was the study for darolutamide. High-risk nonmetastatic CRPC, nonmetastatic on conventional imaging, a PSA doubling time of 10 months or less, randomized 2:1 to darolutamide plus ADT versus placebo plus ADT, and a primary end point of MFS. You can tell that some of the same [investigators] were involved in multiple trials and coordinating the way that they were designed.
Good median age, exactly the same at 74, with a majority of patients having a PSA doubling time that was predominantly less than or equal to 6 months.
The MFS: again a 22-month improvement in MFS [40.4 vs 18.4 months; HR, 0.41; P <.0001]. The OS was associated with a 31% reduction in the risk of death [HR, 0.69; P =.003]5; despite a significant amount of crossover from placebo arm to treatment arm, it could not make up the time. So there was a consistent message with these agents.
AEs were interesting in that falls were similar between darolutamide and placebo, fatigue was only slightly more, maybe numerically but not necessarily statistically significantly more here and otherwise, similar in terms of other AEs.
When we look across the board, we see similar prolongation of MFS and similar prolongation of OS. These agents consistently are demonstrating for us that if we have a [patient with] nonmetastatic CRPC, we may be able to treat them with an AR antagonist for improved MFS. Importantly, despite crossover in the control arm, [there is] improvement in OS, which is not something we necessarily thought that we would see, because these patients with nonmetastatic disease will get multiple additional therapies, including sipuleucel-T and others in the metastatic CRPC setting. And they couldn’t make it up if they weren’t treated early in that nonmetastatic CRPC setting.
What percentage of the study populations were Black, because these are patients who tend to have an aggressive type of cancer?
I haven’t seen the breakdown on these trials. I haven’t looked recently, but in general on these CRPC trials, it’s somewhere usually around 3% to 6%, I think. So particularly for things [such as] the ARAMIS trial, a large number of the patients enrolled were ex-US and in some of the other trials as well. When these large phase 3 trials are coming through the pipeline, there are US sites, but there are so many ex-US sites for various reasons that I’m sure the pharma companies could explain.
How does imaging work into your decision-making for patients such as this?
So I would not order an Axumin or a PSMA [scan] in this setting, unless the patient was interested in pelvic radiation. I would be using that to demonstrate that there’s disease outside of the pelvis that would not benefi t from having pelvic radiation. But if the patient was never interested in pelvic radiation, which this patient was not, I wouldn’t order it.
We don’t have PSMA at Northwestern; we do have Axumin. But I only order it; we have a clinical trial that just completed accrual for which we would use Axumin PET to identify areas of disease, and then we could enroll. This patient, from my perspective, is nonmetastatic CRPC; he fits the inclusion criteria of phase 3 trials that demonstrate not only MFS advantage but also OS advantage. So I would continue my ADT, and I would start an AR antagonist.
How do you choose between these 3 agents?
The differences in these hormonal agents translate to clinical consequences if you’re thinking about things [such as] tolerability toxicity.
In general, at least when we look at the trials, they seem relatively balanced in terms of their AEs. Although comparing drugs with placebo, maybe darolutamide is a little bit less.
There is also some signal that suggests that darolutamide may have fewer drug-drug interactions in addition to having lower blood-brain penetration. So warfarin does have some interactions with apalutamide and enzalutamide. Plavix [clopidogrel] also has some apalutamide and enzalutamide interactions; there are multiple and a lot of them [in] cardiovascular drugs. But it’s hard when we’re trying to prescribe these drugs. These men, many of whom are elderly, have cardiovascular comorbidities, [so] we can have a lot of interactions. It’s nice to have a drug where we send the script and it doesn’t pop back at us to say you need to choose this or adjust your β-blocker or whatever it is.
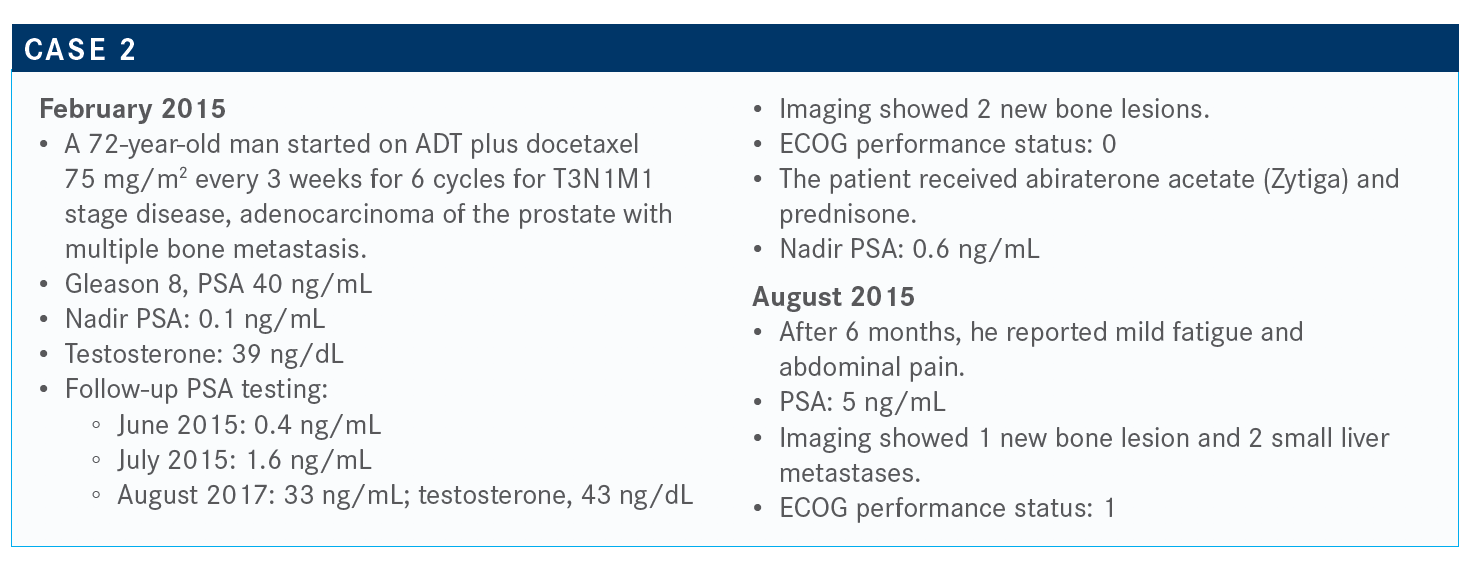
What is your approach and that of your institution to genetic testing in patients with prostate cancer?
We’re still relying on [tissue biopsies over liquid biopsies]. That’s where the majority of data are. It’s interesting and concerning that sometimes there are these discrepancies in liquid biopsies in terms of their results.
I think that’s where we’re going as a field, as those liquid biopsies, hopefully, become more viable and more valid; then we can do multiple liquid biopsies and have the same results for multiple tests. I think we’re going in that direction because [getting tissue for biopsies is] a problem in prostate cancer that we definitely need to overcome.
If this patient had widespread bone metastases, would you consider adding radium-223 (Xofigo)?
This is clearly within the indication of radium-223. For using radium, we would never want to use radium in the setting [of visceral metastases].
In the ERA 223 study, we all saw that in a concerning combination of radium plus abiraterone versus abiraterone alone, there were more fractures in the combination arm.6 But if we used an osteoclastinhibiting agent [such as] zoledronic acid [Reclast] or denosumab [Xgeva], we could avoid those complications or stop that from happening.
I’ve definitely used radium before chemotherapy. I’ve definitely used chemotherapy before radium. But there are certain people [who] don’t want to have hair loss, because whatever they do for their living, they feel tied to their hair. It’s not just a woman thing. They say, “Well, I’m an investment banker, and if people see me lose all my hair, they’re not going to be confident in my ability to maintain their long-term financials.” But I am very respectful and try to give them options that may allow them to not lose their hair. So I have used this, certainly before chemotherapy.
I think radium is similar, in my experience, to sipuleucel-T sometimes in the way that there seems to be some people who really benefit and other people who I think this was not especially effective for them. But it’s hard to tell on the front end sometimes who those people are going to be.
Do you use bone pain as an indicator for treatment?
Absolutely not. So, radium, from my perspective, as do all of our therapies for metastatic CRPC that are currently approved, prolongs survival. And yes, the indications suggest that we should [go based off ] symptomatic disease, but what if that’s fatigue because of anemia? There can be other symptoms that can be there.
In a European expanded access program, patients who were asymptomatic had a much better survival related to their exposure to radium than those patients who were symptomatic. And perhaps it’s because they did get more cycles, but again, it’s this whole concept that the earlier we use whatever treatment we use, it’s going to be more effective.
So, no, I’m not necessarily wedded [to radium], and ultimately, if I don’t have a clear benefit of 1 treatment over another, I try to understand what the patient wants, and I try to push my collaborating doctors to say, “Hey, this is what the patient wants. There’s a survival advantage; let’s go forward.” That’s just my take.
What treatment options are available at this point?
The CARD trial,7 I think everybody is familiar with. [This study looked at] patients with metastatic CRPC who had already been exposed to docetaxel chemotherapy who had progression of disease on an AR-targeted agent. They had to have progressed within 12 months or less on whatever that AR-targeted agent was. Then they were randomized to treatment with cabazitaxel [Jevtana] chemotherapy or the other AR-targeted agents that are approved for the metastatic CPRC setting—enzalutamide or apalutamide, whatever they hadn’t had before. They were followed for a primary end point of radiographic progression-free survival [rPFS], but also followed for things [such as] OS and other secondary end points. They were well balanced between the 2 groups.
The rPFS strongly favored cabazitaxel rather than that second AR-targeted agent [8.0 vs 3.7 months; HR, 0.54; P <.0001]. Interestingly, and importantly, the OS also favored cabazitaxel [13.6 vs 11.0 months; HR, 0.64; P =.0078], which, from my perspective, not only demonstrated the benefit of cabazitaxel but also demonstrated that there’s a relatively limited benefit to a second AR-targeted agent after initial progression on a first AR-targeted agent.
The AEs leading to treatment discontinuation were higher in the cabazitaxel arm, as we might expect. There were no unexplained or unexpected signals in terms of toxicity associated with cabazitaxel in the study.
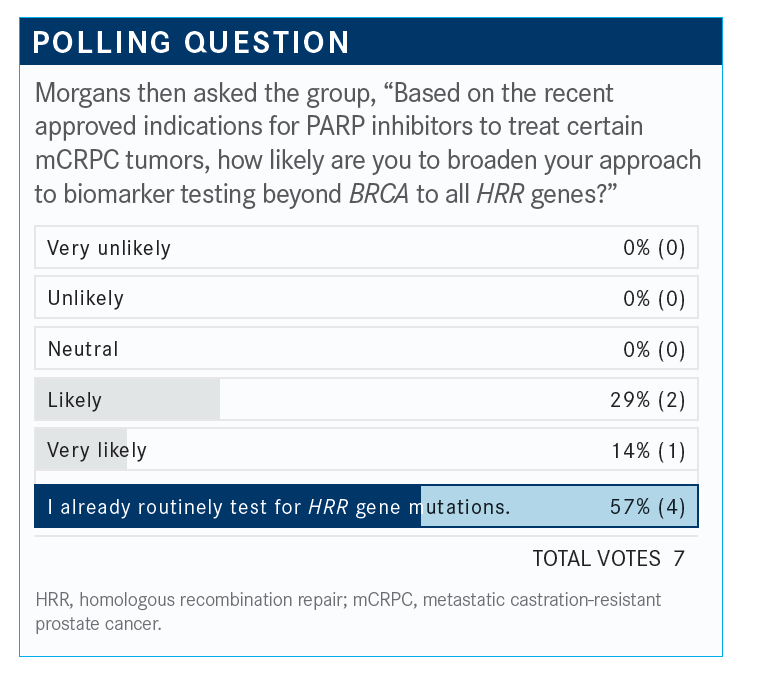
Which treatments have necessitated the use of expanded HRR gene mutation testing?
TRITON28 is the phase 2 study that led to the approval of rucaparib [Rubraca], and this is a study that looked for patients who had these HRR [homologous recombination repair] genes that were mentioned. They all had mCRPC [metastatic CRPC]. They had to have these mutations, at least in germline testing, they had to have progression on abiraterone, apalutamide, or enzalutamide. They also had to have a prior taxane and they had to have a good performance status. They were treated with rucaparib 600 mg twice a day and they were monitored for their progression.
The overall response rate in selected patients was 43.5%.9 And then their PSA response, in that overall efficacy population, was 54.8%. We did see a fair number of patients having a partial response and some patients even having a complete response in this setting.
I’ll emphasize [that safety was analyzed in patients with] BRCA1 or BRCA2. They had a relatively tolerable toxicity profile. Most [95.3%] had at least 1 AE, but in terms of grade 3 or greater, AEs there were about half [52.9%] that did, but most of these did not lead to treatment discontinuation [5.9%].
These treatment-emergent AEs were as we would expect, mostly things [such as] cytopenias, anemia, and thrombocytopenia. Some fatigue and gastrointestinal upset [also were seen]. So the FDA has granted the approval for rucaparib based on the phase 2 trial,10 which is exciting, in this mCRPC setting for only [patients with BRCA1 or BRCA2 mutations].
PROfound11 is a phase 3 trial also with [patients with] mCRPC who had progressed on apalutamide or enzalutamide—or any other nonhormonal antiandrogen that was approved at that time, [most often including] abiraterone, as well as potentially a taxane chemotherapy, though not all had exposure. These patients were split into 2 cohorts: BRCA1, BRCA2, or ATM mutants or other alterations, which was cohort B. And they were randomized to olaparib [Lynparza] versus the alternative AR-targeted agents. We were following these patients for rPFS.
We saw that rPFS was longer in cohort A, which again was BRCA1, BRCA2, or ATM in those patients who had olaparib as compared with the alternative AR-targeted agent [7.4 vs 3.6 months; HR, 0.34; P <.001]. Again, demonstrating that that secondary AT-targeting agent—and this is not a population that had to progress within 12 months—was not effective.
When we combine these 2 cohorts, the benefit was a little less robust [5.8 vs 3.5 months; HR, 0.49; P <.001]. But again, it didn’t disappear.
Safety summary is as we would expect, based on olaparib use in other populations; most experienced an AE but most did not lead to discontinuation. These AEs are predominantly anemia, some nausea, and other cytopenias.
So this was approved for anyone with an HRR gene–mutated metastatic CRPC.12 And this is after any AR-targeted agent; you do not have to be exposed to a prior taxane.
So if you had a patient who had progressed on an AR-targeted agent and a taxane and had a BRCA2 mutation, which agent would you choose between these 2 PARP inhibitors?
Rucaparib was approved fi rst, but it was…neck and neck for these 2 drugs. So it doesn’t make a whole lot of diff erence in my treatment decisions. Olaparib is in more studies, TRITON2 is only 1, although TRITON3 is ongoing, but olaparib was approved [based] on a phase 3 [trial] versus rucaparib, which was approved on a phase 2. I guess we’ll have to see the phase 3 for rucaparib to see where things stand. And I think we’re all eager to understand where that goes.
References:
1. Sternberg CN, Fizazi K, Saad F, et al; PROSPER Investigators. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382(23):2197-2206. doi:10.1056/NEJMoa2003892
2. Smith MR, Saad F, Chowdhury S, et al; SPARTAN Investigators. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408-1418. doi:10.1056/NEJMoa1715546
3. Fizazi K, Shore N, Tammela TL, et al; ARAMIS Investigators. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235-1246. doi:10.1056/NEJMoa1815671
4. Small EJ, Saad F, Chowdhury S, et al. Final survival results from SPARTAN, a phase III study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(suppl 15):5516. doi:10.1200/JCO.2020.38.15_suppl.5516
5. Fizazi K, Shore ND, Tammela T, et al. Overall survival (OS) results of phase III ARAMIS study of darolutamide (DARO) added to androgen deprivation therapy (ADT) for nonmetastatic castration-resistant prostate cancer (nmCRPC). J Clin Oncol. 2020;38(suppl):5514. doi:10.1200/JCO.2020.38.15_suppl.5514
6. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
7. de Wit R, Kramer G, Eymard J, et al. CARD: Randomized, open-label study of cabazitaxel (CBZ) vs abiraterone (ABI) or enzalutamide (ENZ) in metastatic castration-resistant prostate cancer (mCRPC). Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394.040
8. Abida W, Bryce AH, Vogelzang NJ, et al. Preliminary results from TRITON2: a phase II study of rucaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination repair (HRR) gene alterations. Ann Oncol. 2018;29(suppl 8):viii271-viii302. doi:10.1093/annonc/mdy284
9. Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castrationresistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. Published online August 14, 2020. J Clin Oncol. doi:10.1200/JCO.20.01035
10. FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castrationresistant prostate cancer. FDA. May 15, 2020. Accessed October 29, 2020. https://bit.ly/2HGYMcy
11. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382:2091-2102. doi:10.1056/NEJMoa1911440
12. FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA. Updated May 19, 2020. Accessed October 29, 2020. https://bit.ly/3jIugw6