Molecular Aberrations May Expand Choices in Cholangiocarcinoma
During his presentation at the Cleveland Clinic Cholangiocarcinoma Symposium, Davendra P.S. Sohal, MD, MPH, reviewed promising novel therapies and those that recently gained indications for patients with cholangiocarcinoma.
Davendra P.S. Sohal, MD, MPH
Davendra P.S. Sohal, MD, MPH
There may be more options for patients with cholangiocarcinoma outside of the few therapies recognized as standard of care. Drugs in late-phase clinic trials, those with indications in other tumors types, and agents with tumor-agnostic approvals offer possibilities for patients with this disease.1
During his presentation at the Cleveland Clinic Cholangiocarcinoma Symposium, Davendra P.S. Sohal, MD, MPH, reviewed promising novel therapies and those that recently gained indications for this patient population.
“For metastatic diseaseand that is how many of our patients present—the standard still remains the 2 chemotherapy drugs: gemcitabine and cisplatin,” Sohal, director of the Clinical Genomics Program at Cleveland Clinic’s Taussig Cancer Center in Ohio, told the audience.
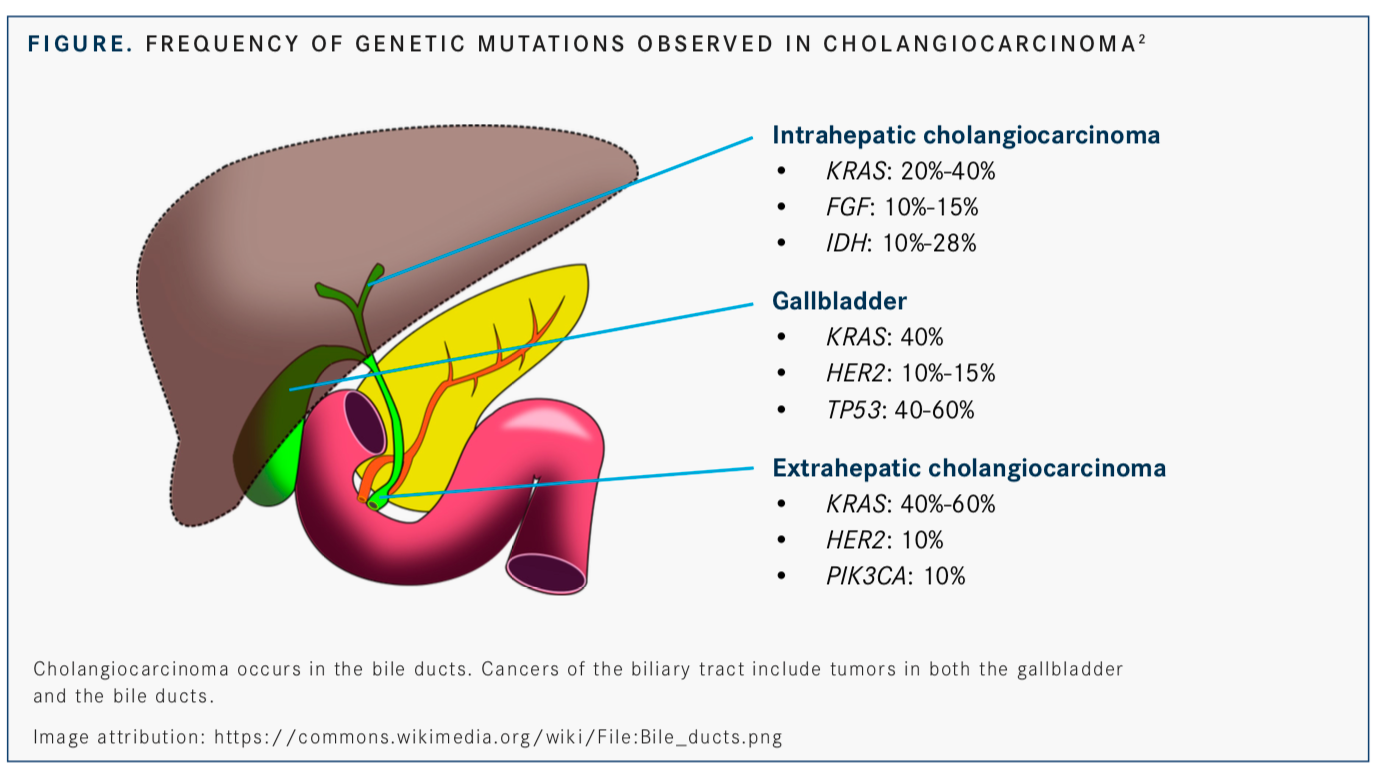
However, some tumors may harbor mutations that could help identify an available regimen. Intrahepatic cholangiocarcinoma tumors are best for identifying genomic and molecular alterations that may be susceptible to treatment with targeted therapies. “KRASis one of these mutations, although it is not druggable,” he said.
Other mutations, includingFGFfusions andIDHalterations, may be the most druggable targets for this tumor type beyond chemotherapy. Each of these 2 aberrations occurs in at least 10% of patients with this type of cancer.2
Extrahepatic disease has a different profile, with small numbers ofHER2andPIK3CAmutations observed in addition to high numbers of mutations inKRAS. Similar numbers ofHER2andKRASmutations are seen, as well as high numbers ofTP53mutations, in gallbladder cancers (FIGURE).2
Agents Without Indications in Cholangiocarcinoma
“Ultimately, every tumor is its own tumor, and we should sequence everything to find out what the tumor’s genetic makeup is. If a drug is applicable, let’s use it,” Sohal said.Sohal referenced preliminary results of MyPathway, a basket trial that is evaluating agents that targetHER2, BRAF, EGFR, or the Hedgehog pathway in non-indicated tumors with relevant molecular alterations. In data presented at the 2019 American Society of Clinical Oncology Annual Meeting, the combination of trastuzumab (Herceptin) and pertuzumab (Perjeta) led to a 50% overall response rate and a 100% clinical benefit rate in patients with HER2-amplified orHER2-overexpressing biliary tract cancer.3
“I doubt this will ever become a labeled [indication] from the FDAusing trastuzumab and pertuzumab for HER2-altered cholangiocarcinoma,” Sohal said. “This just highlights that we sequence it and try to get patients these drugs on clinical trials or otherwise as possible.”
Sohal described the case of a patient who was not eligible to be treated on a clinical trial due to an elevated bilirubin level of >30 mg/dL. “This points to another problem in cholangiocarcinoma,” Sohal said. “Most clinical trials require [patients to have] normal bilirubin levels, and that rarely ever happens in this disease.”
Upon sequencing, it was revealed that the tumor harbored aBRAFV600E mutation, which Sohal called “imminently targetable” based on the availability of agents approved to treat patients with other tumor types that harbor this mutation. A genomics tumor board reviewed the case and determined that the patient could receive the BRAF/MEK inhibitor combination dabrafenib (Tafinlar) and trametinib (Mekinist) with the guidance of a specialist who frequently prescribes the regimen for patients with melanoma.
“[The patient] had a remarkable response, and her bilirubin stabilized within 2.5 months,” Sohal said. The response lasted for 18 months total and survival time was 2.5 years from diagnosis.
“This is an example of sequencing the tumor to find something that is druggable. Even when it is not possible on a clinical trial, sometimes we find that off-label use of a melanoma or lung cancer drug [can be effective],” said Sohal.
Regimens Under Investigation
The phase III ClarIDHy trial of ivosidenib (Tibsovo), an IHD1 inhibitor with FDA approval for treating certain patients with acute myeloid leukemia, is being conducted in patients with previously treated cholangiocarcinoma and anIDH1mutation. In May, Agios Pharmaceuticals, Inc, the company manufacturing the drug, announced that the trial met its primary endpoint of progression-free survival (PFS) superiority over placebo.
“This might be the first targeted therapy approved in cholangiocarcinoma based on a clinical trial,” Sohal said. Data reported at the ESMO Conference 2019 showed median PFS was 2.7 months for patients treated with ivosidenib compared with 1.4 months with placebo (HR 0.37; 95% CI, 0.25-0.54;P<.001).
The median PFS rate at 6-months was 32.0% with ivosidenib, while no patients randomized to placebo were free from progression at this timepoint.4
Another prime target for intrahepatic cholangiocarcinoma includesFGFRfusions, which are present in up to 15% of cases.2
In a phase II trial on the use of the selective pan-FGFR kinase inhibitor infigratinib (BGJ398) in patients with cholangiocarcinoma andFGFR2fusions or translocations, the drug achieved a disease control rate (DCR) of 83.6% (95% CI, 72.5%-91.5%).5
At the ESMO Conference 2019, the FIGHT-202 trial showed an objective response rate (ORR) in the 107 patients withFGFR2fusions/rearrangements (cohort A) was 35.5% and the median duration of response was 7.5 months. In contrast, there were no responses observed in the 20 patients enrolled with otherFGF/FGFRgenetic alterations (cohort B) or in the 18 patients with noFGF/FGFRalterations (cohort C).6
Future Directions
Looking at other plausible biomarkers for therapy in patients with cholangiocarcinoma, Sohal said that microsatellite instability andNTRKgene fusions are targets for the PD-l inhibitor pembrolizumab (Keytruda) and the pan-ALK/TRK/ROS1 inhibitor larotrectinib (Vitrakvi), respectively. More recently, entrectinib (Rozlytrek) received FDA approval for a tumor-agnostic indication across all solid tumors harboringNTRKfusions.7
Another phase III trial that is ongoing, SWOG 1815 (NCT03768414), is looking at a more aggressive chemotherapy option of gemcitabine, cisplatin, and nab-paclitaxel (Abraxane) versus standard gemcitabine and cisplatin. Sohal said this regimen could lead to more toxicities but, hopefully, represents a new option if it can extend survival.
“Ultimately, the take-home message is... if [a] clinical trial is an option, participation should be considered. Please also consider sequencing the tumor,” he concluded. “If we find a target, we [may] have clinical trials to get patients on and make some progress.”
References
- Sohal D. Targeted therapies in cholangiocarcinoma. Presented at: Cleve- land Clinic Cholangiocarcinoma Symposium; July 12, 2019; Cleveland, OH. 2. Sohal DPS, Shrotriya S, Abazeed M, Cruise M, Khorana A. Molecular characteristics of biliary tract cancer.Crit Rev Oncol Hematol. 2016;107:111- 118. doi: 10.1016/j.critrevonc.2016.08.013.
- Hainsworth JD, Meric-Bernstam F, Swanton C, et al. Targeted therapies for advanced solid tumors based on molecular profiles: early results from MyPathway, an open-label, phase IIa umbrella basket trial.J Clin Oncol. 2016;34(suppl 18; abstr LBA11511). doi: 10.1200/JCO.2016.34.18_suppl.LBA11511.
- Abou-Alfa GK, Mercade M, Javle M, et al. ClarIDHy: A global, phase 3, randomized, double-blind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Presented at: ESMO Conference 2019. September 27-October 1, 2019. Barcelona, Spain. Abstract LBA10_PR.
- Javle M, Kelley RK, Roychowdhury S, et al. Updated results from a phase II study of infigratinib (BGJ398), a selective pan-FGFR kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma containing FGFR2 fusions.Ann Oncol. 2018;29(suppl 8; abstr LBA28). doi: 10.1093/ annonc/mdy424.030.
- Vogel A, Sahai V, Hollebecque A, et al. FIGHT-202: a phase 2 study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA). Presented at: 2019 ESMO Congress; September 27-October 1, 2019; Barcelona, Spain. Abstract LBA40. 7. FDA approves entrectinib for NTRK solid tumors and ROS-1 NSCLC. Silver Springs, MD: FDA; August 15, 2019. bit.ly/2ZzlkD1. Accessed August 29, 2019.