Expert Predicts Immunotherapy Combos Will Transform Treatment in Multiple Myeloma
<br /> In the future, we’re going to do combination therapies. They will be used in a subset of patients better defined by profiling,” Kenneth C. Anderson, MD, told an audience at the Charlotte Plasma Cell Disorder Congress in North Carolina.
Kenneth C. Anderson, MD

Kenneth C. Anderson, MD
Combinations of new multiple myeloma therapy approaches, such as monoclonal antibodies, immunomodulatory agents (IMiDs), immunotoxins, bispecific T-cell engagers (BiTEs), and chimeric antigen receptor (CAR) T-cell therapies, are poised to redefine the treatment paradigm.
“In the future, we’re going to do combination therapies. They will be used in a subset of patients better defined by profiling,” Kenneth C. Anderson, MD, told an audience at the Charlotte Plasma Cell Disorder Congress in North Carolina. “If we’re going to have long-term disease-free survivalcure, we have to get minimal residual disease [MRD] negativity with our targeted therapies, and then we need to restore host immunity. We need to have no disease, and we need to have patients off therapy. Quality of life is just as important as having no disease.”
With 24 agents approved in myeloma, 2 within the last year alone, median survival has improved significantly from 3 years to at least 8 to 10 years, according to Anderson, director of the Jerome Lipper Multiple Myeloma Center at Dana-Farber Cancer Institute and the Kraft Family Professor of Medicine at Harvard Medical School, both in Boston, Massachusetts. With these advances, multiple myeloma has become more of a chronic illness for many patients, he added.1
Vaccines Make a Comeback
In the future, immunologic-based interventions, such as vaccines, will be used early in the treatment sequence to delete abnormal clones in precursor conditions that often lead to myeloma, Anderson said. Currently, vaccine cocktails composed of immunogenic HLA-A2specific XBP1, CD138, and CS1 peptides are used to induce multiple myeloma–specific and HLA-restricted cytotoxic T-lymphocyte responses in smoldering myeloma.
“If you vaccinate with a cocktail of peptides, you could get immune responses in all the patients we vaccinate, but I believe we need to use combinations. Therefore, we went on to add lenalidomide [Revlimid] to those vaccinations to augment these immune responses,” Anderson said. “This vaccine works just like any other vaccine that you might give for tetanus or the flu. You can mount in patients a central memory immune response against their own tumor.”
Patterns of Progression May Emerge
Anderson also predicted that the capability to prognosticate will be available in the not-too-distant future. Studying the microenvironment can reveal patterns responsible for clonal evolution within the myeloma cell and, subsequently, disease progression. Single-cell sequencing can detect active or inactive cells correlated with progression. Detecting patients with clonal evolution could allow abnormal clones to be deleted at a very early stage.
“I believe we will have 2 categories. One may be called early multiple myeloma, where the tumor and the microenvironment shows progression without clone evolution, and in my opinion, we are going to treat them as early multiple myeloma,” Anderson explained. “In contrast, in those patients who have a tumor and bone marrow profile, patients who have clonal evolution with a longer timewe will call them monoclonal gammopathy of undetermined significance—in those patients, we will use IMiD-based interventions.”
Quadruplets May Be Key in Newly Diagnosed Disease
Transplant Eligible
In terms of the initial management of transplant-eligible patients with multiple myeloma, there are 3 preferred regimens: lenalidomide (Revlimid), bortezomib (Velcade), and dexamethasone (RVd); cyclophosphamide, bortezomib, and dexamethasone (CyBorD); and carfilzomib (Kyprolis), lenalidomide, and dexamethasone (KRd). However, quadruplet regimens will eventually become the standard, said Anderson.
“In the area of immunotherapies, triplets such as RVd will be supplemented by monoclonal anti-bodiesdaratumumab [Darzalex] in particular—in transplant candidates,” he added, based on data from the phase II GRIFFIN trial.2 Updated results showed that adding daratumumab to RVd induced a stringent complete response rate of 42.4% versus 32.0% with RVd alone (OR, 1.57; 95% CI, 0.87-2.82;P= .1359). Additional data were presented at the 17th International Myeloma Workshop.3
Transplant Ineligible
For newly diagnosed patients who are ineligible for transplant, the use of triplet regimens at attenuated dosing schedules is preferred. Doublets such as bortezomib and dexamethasone or lenalidomide and dexamethasone are often given to frail patients. Again, however, quadruplet regimens, such as “RVd-lite” with or without daratumumab or a newer monoclonal antibody, isatuximab, are slated to become standard.
A phase I study showed that isatuximab, when given up front with RVd in transplant-ineligible patients with newly diagnosed myeloma, produced an overall response rate (ORR) of 100%; a very good partial response or better was achieved in 92% of efficacy-evaluable patients.4 Moreover, 44% of MRD-evaluable patients achieved MRD negativity.
Options Abound in Relapsed/ Refractory Disease
In the relapsed setting, several monoclonal antibodies are available for use in combination regimens. “We’re lucky in myeloma. We have an armamentarium to choose from, and we can consider: What has been the prior exposure? What are the factors in the patient that might inform the best treatment?” Anderson said. “I’ll just remind you that when we use antibodies in relapsed myeloma, there are often durable responses.”
Elotuzumab (Empliciti) in combination with lenalidomide and dexamethasone showed a median OS advantage compared with lenalidomide/ dexamethasone alone in patients with relapsed/ refractory disease at 4 years (48.3 months vs 39.6 months; HR, 0.78; 95% CI, 0.63-0.96).5
Combining elotuzumab with pomalidomide has also demonstrated a 46% reduction in the risk of progression or death when including patients resistant to lenalidomide and bortezomib.6
Another combination, daratumumab plus lenalidomide and dexamethasone, induced deeper responses than lenalidomide and dexamethasone alone in patients with relapsed disease, according to updated data from the phase III POLLUX trial.7
The Future Looks Bright
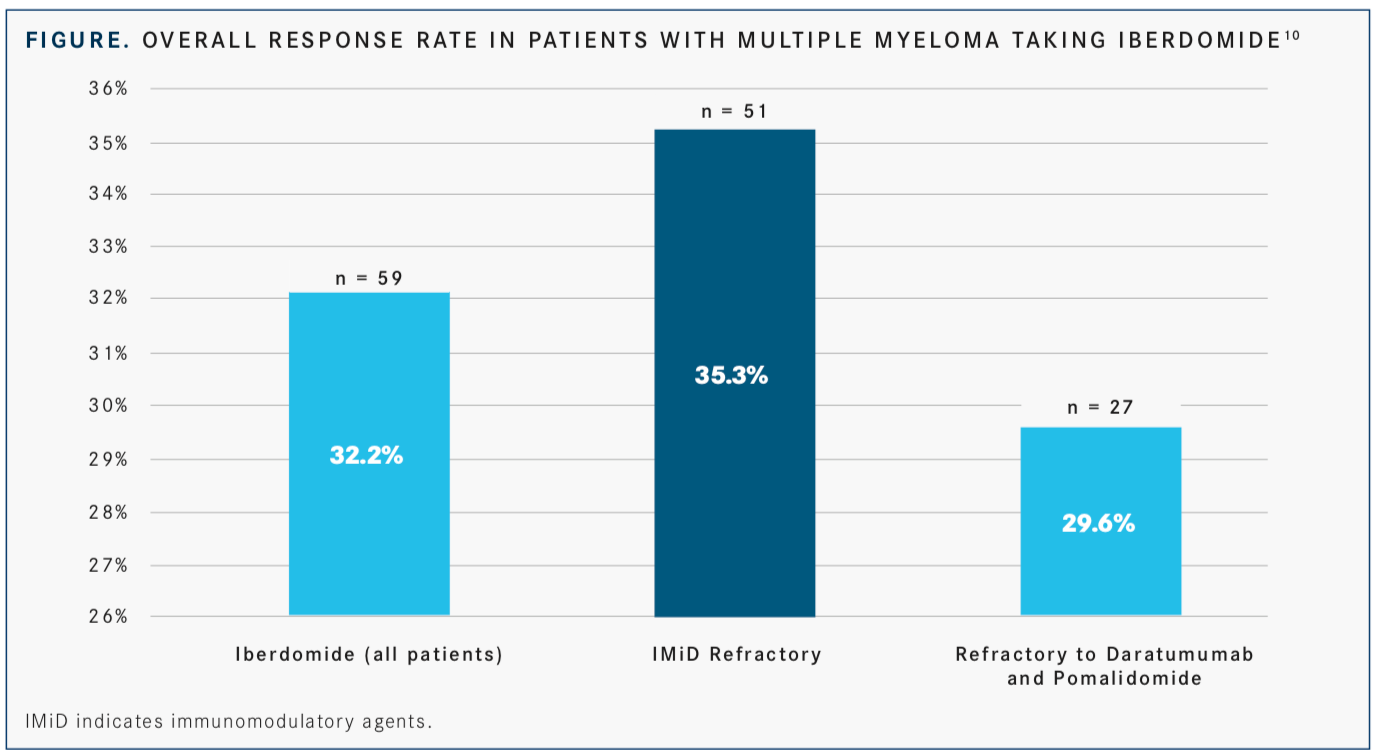
Lastly, data from the phase III ICARIA-MM trial showed that isatuximab in combination with pomalidomide (Pomalyst) and low-dose dexamethasone led to a >40% reduction in the risk of disease progression or death compared with Pd alone in this patient population.8Furthermore, median PFS was 11.53 months with the combination versus 6.47 months with Pd alone (HR, 0.596; 95% CI, 0.436-0.814). Based on these data, the FDA accepted a biologics license application for the agent for use in this setting.9Investigators are exploring iberdomide in combination with dexamethasone in a phase I trial, results of which were presented at the 2019 American Society of Clinical Oncology Annual Meeting. The ORR among evaluable patients (n = 59) with iberdomide was 32.2%. Those who were IMiD refractory (n = 51) had an ORR of 35.3% with the investigational doublet, whereas patients who were refractory to daratumumab and pomalidomide (n = 27) had an ORR of 29.6% (FIGURE).10
Immunotoxins also show promise. The B-cell maturation antigen (BCMA)targeting auristatin immunotoxin, GSK2857916, demonstrated a 60% ORR with an updated median PFS of 12 months in heavily pretreated patients with relapsed/ refractory disease.11Overall, the agent was well tolerated, with a manageable toxicity profile.
Even though BiTEs are available for use in lymphomas and leukemias, there has yet to be an approval in myeloma. However, 5 companies are evaluating these agents in this setting, Anderson said, adding that the furthest along in the developmental process is AMG 701.
A lot of work is also being done with CAR T cells in myeloma, Anderson said. Most notable is the product bb2121, which has been shown to induce deep and durable responses in heavily pretreated patients with relapsed/refractory disease. Specifically, results for the first 33 patients who received the agent showed an ORR of 85%; 45% (n = 15) achieved complete responses. Furthermore, median PFS was 11.8 months with the agent (95% CI, 6.2-17.8).12
More strategies with BCMA cellular therapies include potentially expanding the T cells in the presence of a PI3K inhibitor, introducing RNA chimeric antigen receptor products, peptide-stimulated T cells with a vaccine, and combinations composed of vaccination, IMiDs, and checkpoint inhibitors to prevent T-cell exhaustion and prolong response.
“In the future, we will be treating patients with RVd and a monoclonal antibody and we’ll achieve MRD negativity, but that’s not going to be enough,” Anderson warned. “We’re going to have to do something elsesome new form of adoptive immunotherapy—which is going to be designed to correct the problem in the host. We’re only treating half the problem, so stay tuned.”
References
- Anderson KC. Future of immunotherapy in multiple myeloma. Presented at: Charlotte Plasma Cell Disorder Congress; August 9-11, 2019; Charlotte, NC.
- Genmab announces positive topline results in the phase II GRIFFIN study of transplant eligible, newly diagnosed patients with multiple myeloma treat- ed with daratumumab in combination with lenalidomide, bortezomib, and dexamethasone [news release]. Copenhagen, Denmark: Genmab; July 8, 2019. bit.ly/32ffENb. Accessed July 8, 2019.
- Voorhees P, Kaufman JF, Laubach J, et al. Daratumumab + Lenalidomide, Bortezomib & Dexamethasone Improves Depth of Response in Transplant-eligible Newly Diagnosed Multiple Myeloma: GRIFFIN. Presented at: 17th International Myeloma Workshop; September 12-15, 2019; Boston, MA. Abstract OAB-87.
- Ocio EM, Bringhen S. Oliva S, et al. Preliminary results from a phase I study of isatuximab (ISA) in combination with bortezomib, lenalidomide, dexamethasone (VRd), and in patients with newly diagnosed multiple myeloma (NDMM) noneligible for transplant.Blood. 2018;132(suppl 1; abstr 595). doi: 10.1182/blood-2018-99-111244.
- Dimopoulos MA, Lonial S, Betts KA, et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial.Cancer. 2018;124(20):4032-4043. doi: 10.1002/cncr.31680.
- Dimopoulos MA, Dytfeld D, Grosicki S, et al. Elotuzumab plus po- malidomide and dexamethasone for multiple myeloma.N Eng J Med.2018;379(19):1811-1822. doi: 10.1056/NEJMoa1805762.
- Dimopoulos MA, White DJ, Benboubker L, et al. Daratumumab, lenalidomide, and dexamethasone (DRd) versus lenalidomide and dexamethasone (Rd) in relapsed or refractory multiple myeloma (RRMM): updated efficacy and safety analysis of Pollux.Blood. 2017;130(suppl ; abstr 739). bit. ly/2H2NMCY.
- Richardson PG, Attal M, Rajkumar SV, et al. A phase III randomized, open label, multicenter study comparing isatuximab, pomalidomide, and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed/refractory multiple myeloma (RRMM).J Clin Oncol.2019;37(suppl 15; abstr 8004). doi: 10.1200/JCO.2019.37.15_suppl.8004.
- Sanofi: FDA to review isatuximab as a potential treatment for relapsed/ refractory multiple myeloma [news release]. Paris, France: Sanofi; July 10, 2019. bit.ly/2JrKs63. Accessed September 3, 2019.
- Lonial S, van de Donk NWCJ, Popat R, et al. First clinical (phase 1b/2a) study of iberdomide (CC-220; IBER, a CELMoD, in combination with dexamethasone (DEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM).J Clin Oncol.2019;37(suppl 15; abstr 8006). doi: 10.1200/JCO.2019.37.15_suppl.8006.
- Trudel S, Lendvai N, Popat R, et al. Deep and durable responses in patients (Pts) with relapsed/refractory multiple myeloma (MM) treated with monotherapy GSK2857916, an antibody drug conjugate against B cell maturation antigen (BCMA): preliminary results from part 2 of study BMA117159.Blood.2017;130(suppl 1; abstr 741). bit.ly/2luUIkE.
- Raje N, Berdeja J, Lin Y, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma.N Eng J Med.2019;380(18):1726- 1737. doi: 10.1056/NEJMoa1817226.
Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More