Lenvatinib Is Effective, Regardless of Age in HCC
No differences in efficacy and safety were seen in a study cohort of 1325 patients undergoing frontline treatment with lenvatinib for unresectable hepatocellular carcinoma.
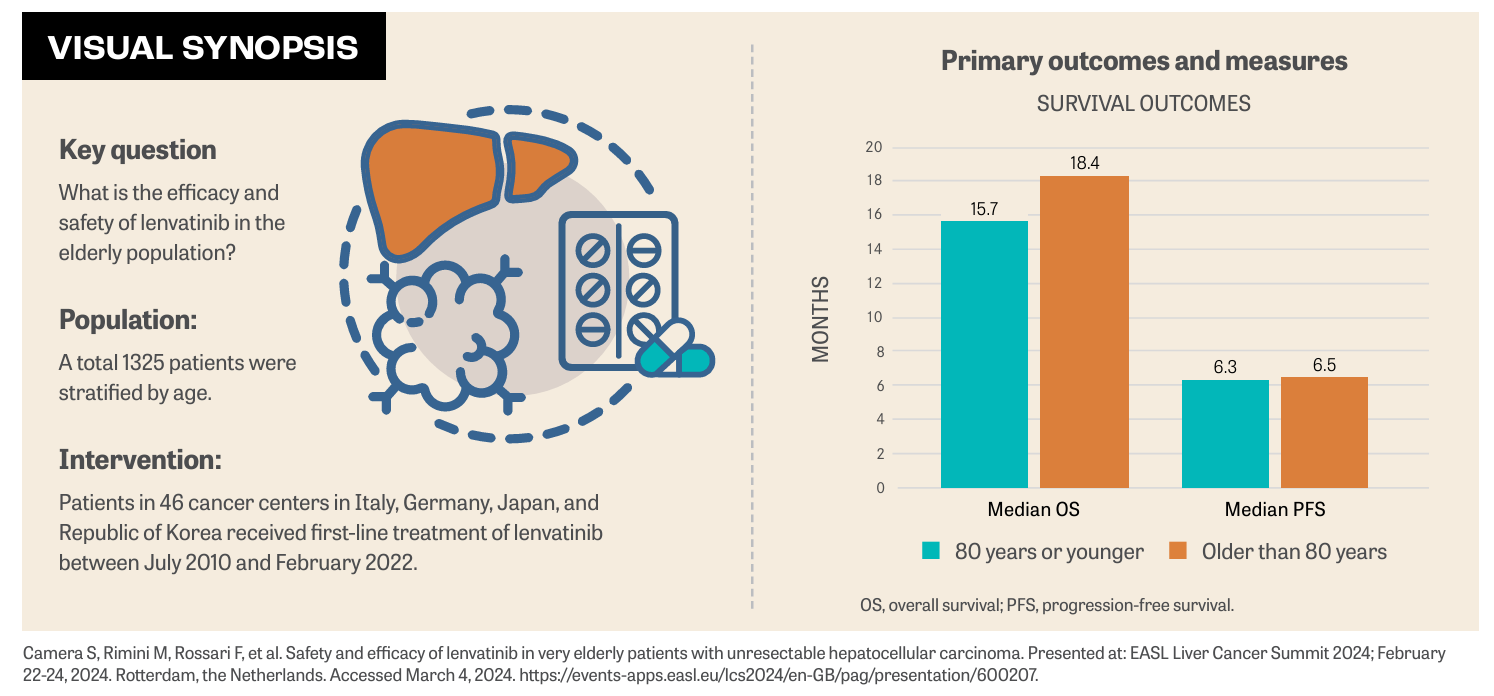
No differences in efficacy and safety were observed in a study cohort of 1325 patients undergoing frontline treatment with lenvatinib (Lenvima) for unresectable hepatocellular carcinoma (HCC). Patients were stratified by age with patients older than 80 vs patients 80 years and younger by survival outcomes, disease control rate, and response rate. Findings were presented at the European Association for the Study of the Liver (EASL): Liver Cancer Summit 2024.1
Median overall survival (OS) was 15.7 months for patients older than 80 years vs patients 80 years and younger, respectively (HR, 1.02; 95% CI, 0.84-1.25; P = .8281), Andrea Casadei Gardini, MD, and coinvestigators presented in a poster during the conference.1
Median progression-free survival (PFS) was 6.3 months for patients older than 80 years vs patients 80 years and younger, respectively (HR, 1.07; 95% CI, 0.91-1.25; P = .3954). No differences between the 2 study groups were found in terms of disease control rate (80.8% vs 78.8%; P = .44) and response rate (38.2% vs 37.9%; P = 0.88).1 Data evaluating the use of lenvatinib in very elderly patients are limited, although the incidence of HCC in this patient population is constantly increasing.1 Prior to the current study, a trial evaluated 100 patients with HCC who received lenvatinib were selected using propensity score matching.2 Regarding OS and PFS, there were no significant differences between the elderly and nonelderly groups (HR, 0.972; 95% CI, 0.374-2.529; and HR, 1.362; 95% CI, 0.687-2.700, respectively).2 Tada T, et al reported that elderly patients experienced fatigue (36.0%), decreased appetite (26.0%), and hypothyroidism (24.0%).2
In the current study, investigators reported that patients younger than 80 years experienced significantly more hand-foot skin reactions (grade ≥ 2) and decrease appetite (grade ≥ 2) than patients 80 years and older. Patients 80 years and older experienced significantly more fatigue (grade ≥ 2).1 Researchers noted that Barcelona Clinic Liver Cancer stage was the only independent predictor of OS (HR, 1.59; 95% CI, 1.11-2.29; P = .01115).1
REFERENCES:
1. Camera S, Rimini M, Rossari F, et al. Safety and efficacy of lenvatinib in very elderly patients with unresectable hepatocellular carcinoma. Presented at: EASL Liver Cancer Summit 2024; February 22-24, 2024; Rotterdam, the Netherlands. Accessed March 4, 2024. https://tinyurl.com/35papw5z
2. Tada T, Kumada T, Hiraoka A, et al. Safety and efficacy of lenvatinib in elderly patients with unresectable hepatocellular carcinoma: a multicenter analysis with propensity score matching. Hepatol Res. 2020;50(1):75-83. doi:10.1111/hepr.13427

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More