Langer Discusses Trials in Multiple Settings for Non–Small Cell Lung Cancer
Corey J. Langer, MD, discusses treatment options for a patient with non–small cell lung cancer based on the clinical trial research.
Corey J. Langer, MD

Corey J. Langer, MD, director, Thoracic Oncology, Abramson Cancer Center and professor of Medicine, Hospital of the University of Pennsylvania in Philadelphia, PA, discusses treatment options for a patient with non–small cell lung cancer (NSCLC) based on the clinical trial research.
Targeted OncologyTM: Do you prefer to use carboplatin or cisplatin as adjuvant therapy when you’re treating patients with NSCLC?
LANGER: We should have been doing adjuvant studies with carboplatin. There is a mind-set that carboplatin is a palliative drug—the metastatic stage IV recurrent drug—and cisplatin is the standard in the curable setting. It’s been so difficult to part with that notion. The only trial that used carboplatin/paclitaxel was the CALGB trial [NCT00003117]. Every other trial used cisplatin. I think there’s still an opportunity to remedy that wrong. My rule of thumb [is], if they’re over 70 or 75 years, unless they are extremely fit, I will generally give them a carboplatin combination. If they’re [younger than] 70, I’ll generally go ahead with cisplatin.
The CALGB trial took [the carboplatin] regimen and applied it in the adjuvant setting.1 That’s where we get the 4 [cm] rule. The trial overall was negative, but in the 4 [cm] or bigger group, which is what this patient has—a 5.5-cm tumor—there was a statistically significant survival improvement. The hazard ratio was 0.69 [95% CI, 0.48-0.99; P = .043] with the carboplatin regimen. Even there we do have some, at least post hoc, evidence that it’s not unreasonable.
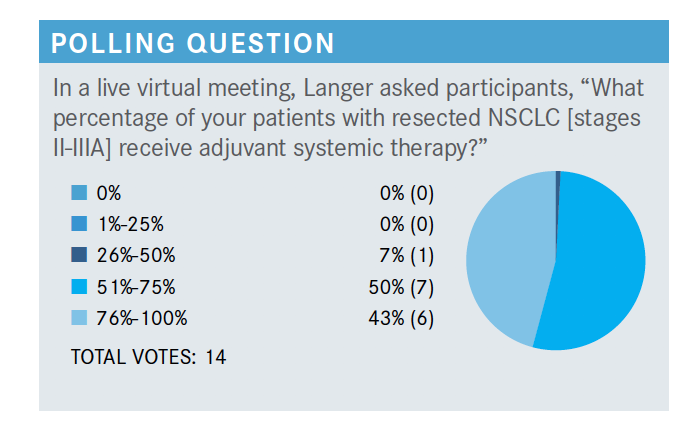
Which parameters are important when deciding on whether to use adjuvant systemic therapy post resection?
I think most of us will consider [the patient’s] node status. Lymphovascular [invasion] puts patients at high risk. The problem is we’ve never really separated that out and proven that adjuvant benefits that group. In my gut, I think those [patients] need it, but intellectually, at least prospectively, we have not [proven it]. Visceral pleura [invasion]—I know it upstages patients, but that in and of itself does not reduce prognosis.
There is the potential for toxicities with renal insufficiency. If somebody has a [ECOG] performance status of 2 or 3, that’s the kind of patient who we’ll hold off on [treating].
What do the guidelines in this setting suggest?
Adjuvant cisplatin-based chemotherapy is recommended for patients with node-positive [disease] and now for patients in stage II with larger lesions—[stage] IB for those [with] high-risk features.2 For the smaller lesions, certainly less than 3 [cm], no adjuvant therapy is recommended. The LACE [Collaborative Group’s] meta-analysis showed a disadvantage to platinum-based treatment in the stage IA group.3 The hazard ratio was 1.40, so that is probably, at least to some extent, harming some of these [patients].
Which trials show relevant data on the use of radiation therapy in this setting?
The LUNG ART trial [NCT00410683] is about 10 years in the making—a randomized trial evaluating the role of radiation in N2 disease.4 These patients’ disease had to be completely resected. They could have received neoadjuvant or adjuvant therapy. They were stratified based on whether they [received] postoperative or preoperative chemotherapy; their node status, multistation versus single station; whether they had a PET; and histology. The primary end point was disease-free survival [DFS]. Secondary end points were overall survival [OS], the pattern of relapse, local failure, second cancers, and toxicity.
The DFS hazard ratio [for postoperative conformal radiotherapy (PORT) vs no PORT] was 0.85 [95% CI, 0.67-1.07], and the P value was not significant [P =.16]. Numerically, it was better; it was 30.5 months versus 22.8 months [for PORT vs the control, respectively], but the differences were not significant. The 3-year DFS rate was not significant nor did it translate into an OS advantage [47.1% for PORT vs 43.8% in the control arm].

How do past trials for adjuvant EGFR-targeted therapy compare for this patient population?
The RADIANT trial [NCT00373425] was an adjuvant trial comparing erlotinib [Tarceva] with placebo in patients with IB-IIIA disease.2 They had to be fluorescence in situ hybridization positive but otherwise EGFR wild-type…or [mutation-positive]. There was a DFS advantage in [the erlotinib] group [with mutations], but, over time, that difference, which was at least initially statistically significant, lost its significance and there was no OS advantage.
Then we participated in the SELECT trial [NCT00567359], which was a phase 2 trial looking at adjuvant erlotinib in resected EGFR-mutant disease; again, larger node-negative tumors, as well as N1-N2 disease. There was an interesting observation. The treatment continued for 2 years, and after that 2-year point, there was an abrupt drop-off in the DFS, suggesting that these drugs may work but [only when patients are taking] them. There was an accelerated pattern of relapse once the drug was stopped. Unsurprisingly, the patients with stage I disease did a bit better than [those with] stage II and IIIA [disease].
The ADJUVANT trial from China [NCT01405079]…took patients strictly with node-positive disease—stage II N1 or IIIA N2—[and] compared gefitinib [Iressa] with vinorelbine/cisplatin. They did not give chemotherapy routinely to all these patients. The vinorelbine/cisplatin was considered the standard. They were hoping to see an advantage for gefitinib and, at least in DFS, they did, but not in OS.
What are the most recent data for this group of patients?
These trials laid the groundwork for ADAURA [NCT02511106], which was presented by Roy Herbst, MD, PhD, at the 2020 virtual American Society of Clinical Oncology [ASCO].5 It had a 1:1 randomization to osimertinib [Tagrisso] or placebo, and basically the same population as the RADIANT trial, with the important caveat that patients had to have an EGFR mutation and either exon 19 or 21 [deletion]. Stratification was by stage, the nature of the mutation, and ethnicity—Asian versus non-Asian. The eligibility criteria [included] adults with fully resected NSCLC. They had a maximum interval between surgery and randomization of 10 weeks for those not getting adjuvant chemotherapy and up to half a year for those who did get adjuvant chemotherapy. The primary end point was DFS with a targeted hazard ratio of 0.70. Secondary end points included DFS in the overall population, not just II and IIIA disease; DFS at specific time points: 2, 3, 4, and 5 years; OS; and health-related quality of life. The treatment continued for a full 3 years, and treatment was stopped for recurrence, completing treatment, toxicity, or patient preference. The trial was unblinded early due to an efficacy benefit, and this [was done] by the independent data monitoring committee. There was an unplanned interim analysis that was presented at ASCO. Fortunately, at least at the unblinding point, the entire study had completed enrollment, and virtually all patients had been followed for up to a year.
The demographics were standard for an EGFR-mutant population. The majority of patients were never smokers. A majority were of Asian ethnicity. All patients had good performance status. There was a fairly even distribution between stages IB, II, and IIIA. Virtually all patients had adenocarcinomas you’d expect with EGFR, and a slight majority had exon 19 mutations. Intriguingly, 40% did not get adjuvant\ chemotherapy, which I think flies in the face of most of our standard practice.
Please discuss the efficacy of the ADAURA trial.
The primary end point looking at DFS in the node-positive population had an amazing hazard ratio, way below 0.7; it was 0.17 [95% CI, 0.12-0.23].5 Looking at the entire population, the hazard ratio is still pretty impressive at 0.21 [95% CI, 0.16-0.28]. The median follow-up [in the interim analysis], at least for the osimertinib group, was just shy of 2 years and about 1 year and 3 months for the placebo group.6
Every subgroup had a DFS benefit with osimertinib, regardless of sex, age, smoking history, or ethnic background. Note that the [stage] IB group, although the hazard ratio was significant, was not as provocative or as pronounced as the node-positive group…at 0.39. Chemotherapy didn’t make a difference, either. If patients [received] adjuvant chemotherapy, they had greater benefit. If they didn’t, there was still a benefit, at 0.23 hazard ratio.
For [stage] IB, the original hazard ratio was 0.50 [95% CI, 0.25-0.96], not quite as impressive as the node-positive group.5 The 2-year disease-free survival rates improved by 56% in the [stage] IIIA group and by 35% in the stage II group, but only by about 14% in the [stage] IB group.
At 2 to 3 years, there was almost complete central nervous system protection.6 The DFS hazard ratio was fairly striking at 0.18 [95% CI, 0.10-0.33]. But there was a drop-off at that 3-year mark.
OS, unsurprisingly, had virtually no difference, but we’re still early.5 The hazard ratio was 0.40 [95% CI, 0.18-0.90], and, fortunately, in both arms the vast majority remain alive.
What was the toxicity profile of osimertinib?
The safety summary had no major shocks. Anyone who has prescribed osimertinib is familiar with this—11% stopped treatment because of an adverse event [AE], and about 7% had to dose reduce. The incidence of grade 3 or higher AEs that was… attributable to osimertinib was about 10% compared with 3% in the placebo group. The usual AEs were diarrhea—although very low incidence of grade 2 or 3; about 16% had paronychia, and there was far lower incidence of standard rash or pruritis. I find that paronychia is probably what you see in terms of cutaneous toxicity with osimertinib. The typical acneiform rash usually isn’t too bad. A fair number had stomatitis, about 18%. I have a lot of patients complaining of…postnasal drip. [There were] some upper gastrointestinal complaints, decreased appetite, and nausea. But…osimertinib is far better tolerated than gefitinib or erlotinib and particularly compared with afatinib [Gilotrif].
In which patient populations would you use osimertinib?
[For patients with stage] IIIA disease, I would probably offer it with at least some enthusiasm. In stage II for N1 disease, [I would] consider it, but [I would] have a nuanced discussion [with the patient]. I’m not sure I’m ready to embrace it in [stage] IB disease yet, based on the hazard ratios and the risk-benefit of each approach. But the [stage] IIIA group is probably at highest risk for brain relapse, and that’s 1 of the more compelling reasons to use it.
REFERENCE:
1. Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26(31):5043-5051. doi:10.1200/JCO.2008.16.4855
2. Spigel DR. Discussion of LBA5. Presented at: 2020 ASCO Virtual Scientific Program; June 1-4, 2020. Accessed November 5, 2020. https://www.bit.ly/38gIEd8
3. Pignon JP, Tribodet H, Scagliotti GV, et al; LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552-3559. doi:10.1200/JCO.2007.13.9030
4. Le Pechoux C, Pourel N, Barlesi F, et al. An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK). Ann Oncol. 2020;31(suppl 4):S1178. doi:10.1016/j.annonc.2020.08.2280
5. Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38(suppl 18):LBA5. doi:10.1200/ JCO.2020.38.18_suppl.LBA5
6. Wu YL, Tsuboi M, He J, et al; ADAURA Investigators. Osimertinib in resected EGFRmutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071