Implications of the Gut Microbiome for Cancer Immunotherapy
The results of multiple studies suggest that bacteria may influence both cancer growth and the immune system, with certain species linked to improved immune surveillance of cancer. Some bacteria interact with the host’s immune system through paracrine factors to shape the immune system’s response to cancer.
Arjun V. Balar, MD
The immune system is very effective against invading pathogens such as viruses and bacteria, as well as cancer cells that arise through genetic mutation. The activity of the immune system must be kept in check, however, to maintain homeostasis and avoid the development of autoimmune conditions. The immunosuppressive mechanisms that accomplish this can be co-opted by cancer to evade immune eradication. Checkpoint inhibitor immunotherapies are a relatively new class of cancer treatments that aid the immune system by targeting specific cell-surface molecules to reactivate antitumor immunity. Unlike the effects of conventional cytotoxic chemotherapy, antitumor responses to checkpoint inhibitors, such as antiPD-1 and anti–PD-L1 antibodies, can be long-lasting, reflecting the unique ability of the immune system to continually adapt to and keep pace with cancer evolution.
The results of multiple studies suggest that bacteria may influence both cancer growth and the immune system, with certain species linked to improved immune surveillance of cancer. Some bacteria interact with the host’s immune system through paracrine factors to shape the immune system’s response to cancer. The gut microbiome represents the largest collection of bacteria in the human body and has been a major focus of research for its effects on cancer formation and prevention.
Specific bacterial species in the gut have been shown to be associated with a favorable tumor-immune microenvironment; therefore, modifying patients’ gut microbiomes may improve their response to cancer immunotherapy. This review aims to summarize recent research on the role of the gut microbiome in response to immunotherapy as well as cancer progression.
Immune System Defenses Against Pathogens and Cancer
The human immune system can adapt to attack foreign antigens, and it relies on defense strategies (namely, the innate and adaptive immune responses) to protect against invading pathogens. These defense mechanisms are crucial to the maintenance of tissue homeostasis and integrity of the organism. Innate immunity refers to the physical structures and nonspecific cellular and chemical defenses, particularly the skin, mucous membranes, phagocytes, and neutrophils, that act as the first line of defense against foreign pathogens.1The adaptive immune system acts as the second line of defense and has the ability to form memory, using antibody molecules and chemicals to carry out responses to particular foreign substances.2It incorporates cell-and antibody-mediated immune responses, involving T helper cells (T4 cells), cytotoxic T cells, T suppressor cells, memory T cells, B lymphocytes, and plasma cells that produce large amounts of specific antibodies. T4 cells release lymphokines to kill germs in infected cells, and they prompt the proliferation of antibodies against the pathogen. T suppressor cells and cytotoxic T cells, which directly kill infected cells, both slow the immune system’s actions after the pathogen has been killed. Memory T cells ensure that if an infection with the same pathogen occurs again, the response will be more efficient and reduced. B lymphocytes directly bind the antigen and destroy it, using T cells to mark the antigens. When a foreign surface is recognized, B cells form plasma cells that secrete large quantities of antibodies into the bloodstream to bind to specific antigens, allowing antibodies to hinder the antigens’ ability to bind to host cells’ receptors. The immune system is, overall, delicately regulated and activated or deactivated primarily by T lymphocytes and other immune cells to maintain a balanced defense.2
Cancer can lead to weakened immune responses, particularly in patients with disseminated or metastatic disease, through multiple mechanisms of immune suppression. Initially, rapidly dividing cancer cells, ostensibly driven by driver genetic alterations, undergo cell death. Under ideal circumstances, the anticancer immune response begins when dying cancer cells release neoantigens (derived from tumor-specific mutated proteins) into the tumor microenvironment, where they are taken up by dendritic cells and B cells. These cells then present the tumor antigens on major histocompatibility complex molecules to naïve CD8+ T cells, which become activated into cytotoxic T cells, infiltrate the tumor microenvironment, bind to the tumor cells, and induce cancer cell death. To impair antitumor immunity, cancer cells and tumor-infiltrating immune cells often overexpress regulatory checkpoint molecules such as PD-L1, a ligand for the PD-1 receptor expressed on the surface of cytotoxic T cells.3Inhibiting the PD-1/PD-L1 interaction can reinvigorate exhausted T cells and generate antitumor immunity. However, multiple other mechanisms of immune suppression exist, as evidenced by reports that only a fraction of patients with advanced malignancies respond to checkpoint inhibition. This highlights the need to better understand other factors, such as the gut microbiome, that contribute to anticancer immune responses and the efficacy of immunotherapeutic drugs.4,5
The interaction between microbiota and cancer has been studied for over a century, dating back to the first experiments in the late 19th century with Coley’s toxin, a mixture of heat-killed Streptococcus pyogenes and Serratia marcescens that served as a cancer vaccine in patients with bone and soft-tissue sarcomas, leading to immune-based responses.6Our understanding has significantly improved since that time, and efforts are now underway to manipulate the host microbiome to improve responses to modern immunotherapy treatments.
Intestinal Bacteria and Immunotherapy Response
The gut microbiota is defined as the community of microorganisms, including viruses and bacteria, that inhabit the digestive tract. The gut microbiota represents arguably the most crucial collection of bacteria in the body, serving multiple homeostatic functions. The most important of these functionsmaintaining bacterial diversity and preventing the colonization of bacteria that can introduce disease—play an important role in immune homeostasis of the intestine and other environments.7For example, the gut secretes immunoglobulin A, which limits the invasiveness of potentially dangerous bacteria. The microbiome also competes with invasive bacteria for resources; therefore, an infectious pathogen from the environment (eg, Clostridium difficile) may thrive if the native microbiota is suppressed by antibiotics.8Similarly, a favorable gut microbiome profile has many benefits for the host organism, including contributing to immune system development and homeostasis as well as promoting antitumor immunity; conversely, an unfavorable profile can contribute to carcinogenesis. Specifically, dysbiosis, or imbalance of the gut microbiota, can have a broad range of effects, including the development of cancer and other diseases (TABLE).
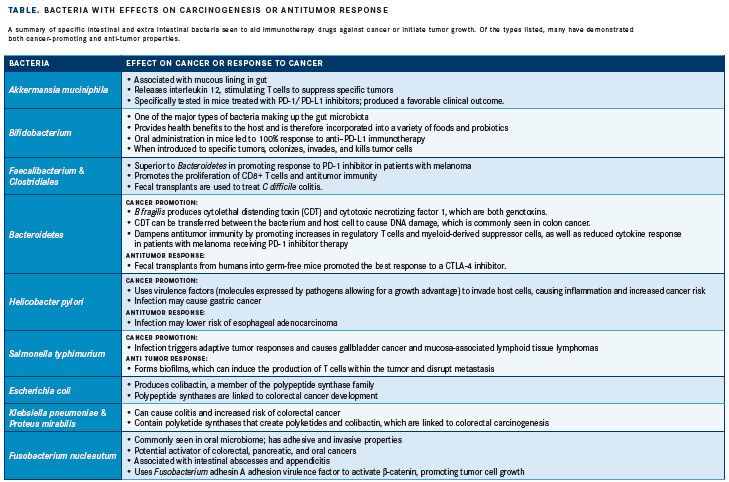
Dysbiosis can induce regulation of certain metabolic activities that lead to cancer. For example, the gut microbiota can synthesize bile acids from fatty tissue, which has been found to lead to hepatocellular carcinoma in mouse models. This synthesis can also influence the metabolism of gonadal hormones, which can increase risk of estrogen-dependent cancer. Certain bacterial species can also contribute directly to the toxicity of certain cancer therapies. For instance, beta-glucuronidase produced by commensal bacteria can reactivate the conversion of the colon cancer drug irinotecan to the active metabolite SN-38 in the intestine, worsening treatment-associated diarrhea.9Defects in the barriers that separate the microbiome from the host can promote carcinogenesis, as they allow for more interactions between the microbiota and the host, permitting transfer to the host of genotoxins and other substances that affect cellular responses to cancer. Targeting the specific bacteria that weaken the barrier may optimize the benefits of immunotherapy in patients with cancer.
Although dysbiosis of the gut bacteria may lead to carcinogenesis, a favorable profile may prevent the development of cancer. For instance, colonic fermentation of otherwise indigestible carbohydrates by gut bacteria generates short-chain fatty acids, which serve to maintain intestinal homeostasis, suppress growth of gram-negative pathogens, and reduce inflammation, all of which protect against colorectal cancer.10A favorable profile can also be important in initiating responses against tumors and assisting immunotherapy drugs in fighting cancer. Diverse gut microbiota and beneficial bacteria, namely Akkermansia muciniphila, initiate the production of immune cell signaling proteins. The composition of the gut microbiome has also been linked to responses to PD-1 inhibitors in patients with advanced melanoma. Patients with microbiomes rich in members of the Faecalibacterium genus and Clostridiales order had improved responses to treatment and longer progression-free survival compared with patients with a higher proportion of the Bacteroidales order. Patients in the former group had more cytotoxic T cells and stronger antitumor immunity, whereas patients in the latter group had a weaker cytokine response and more regulatory T cells.11
Although the gut microbiota may provide antitumor effects independently, research has focused on the coupling of specific gut bacteria and immunotherapy drugs to maximize response. In a retrospective study led by immunologist Laurence Zitvogel, MD, PhD, taking antibiotics had a negative impact on patients’ response to anticancer therapy.12Specifically, 249 patients with lung, kidney, and bladder cancer who were receiving checkpoint inhibitors were retrospectively assessed for concurrent antibiotic use, and the relationship with clinical outcomes was examined. Results from the study indicated that patients who took antibiotics had worse survival than those who did not, suggesting that alteration of the gut microbiome may have influenced the response to checkpoint blockade. Specifically, the authors established that A muciniphila was more prevalent in the gut microbiota of the patients who responded. However, the investigators cautioned that some patients took antibiotics for routine reasons before or soon after starting a PD-1 drug. To further explore the influence of A muciniphila on treatment response, the authors gave germ-free mice (which lack gut bacteria) fecal transplants from patients receiving PD-1 inhibitor therapy. Compared with transplants from nonresponders, transplants from patients who responded well to the therapy produced a better PD-1 inhibitor response in the mice. Moreover, nonresponder mice often became responders after being fed A muciniphila further indicating the effectiveness of this bacterial species in improving the response to immunotherapy. In a separate study led by Jennifer Wargo, MD, patients with melanoma who responded to PD-1 inhibitor therapy were found to have a more diverse microbiome and a larger quantity of specific bacteria than nonresponders. Similar to the Zitvogel study, mice with fecal transplants from responders had better responses to the inhibitor.5 However, in a separate study led by Wargo in patients with melanoma receiving antiPD-1 therapy, analysis of patient fecal microbiome samples (n = 43; 30 responders, 13 nonresponders) showed significantly higher alpha diversity (P<.01) and relative abundance of bacteria of the Ruminococcaceae family (P<.01) in responding patients.13Another study investigated combining manipulation of the gut microbiome with cancer therapy and showed that mice from different laboratories had variation in preexisting immune response to injected tumor cells. Specifically, the studied mice were from 2 different laboratories, Jackson Laboratory (JAX) and Taconic Farms (TAC), and had different commensal microbiota. After being injected with melanoma cells, the JAX mice had a lower tumor growth rate than the TAC mice, which had more aggressive growth. Fecal matter from the JAX mice was then transferred to TAC mice, which led to delayed tumor growth and increased infiltration of cytotoxic CD8+ T cells into tumors. The mice then received PD-L1 inhibitor therapy, and higher efficacy was observed in JAX mice, supporting the finding of improved response to immunotherapy in mice with a favorable gut microbiome. Additionally, in TAC recipients of fecal matter from JAX mice, the PD-L1 inhibitor improved tumor control and circulating T-cell responses. An increase in the abundance of Bifidobacterium species in the microbiome was identified as the cause of the increase in antitumor T-cell response.14Taken together, these data suggest that diversity of gut flora, as well as specific bacteria within the microbiome, can help the immune system both protect against carcinogenesis and generate a more robust anticancer response. Studies currently underway are testing whether clinical outcomes are improved when patients with advanced melanoma receive fecal microbiota transplantation from patients who previously responded to checkpoint inhibitor therapy (NCT03353402 and NCT03341143).
Future Directions
To develop the most effective and individualized cancer therapies, it may be beneficial to investigate the gut microbiome when treating patients with immunotherapy. The overarching goal in terms of the microbiome is eubiosis, or complete bacterial balance, to generate the most favorable mixture of gut bacteria.8The contribution of antibiotic therapy to dysbiosis has led to increased popularity of off-the-shelf probiotic products; however, the potential harmful effects of such products are not yet fully understood. An active area of research involves methods of effectively transferring favorable bacteria while not contributing to dysbiosis. For example, a study found that probiotics intended to reconstitute the gut flora after a course of antibiotics actually delayed the recovery of gut microbiome diversity.15Probiotics that are currently commercially available may have unknown influences on immunity and responses to cancer immunotherapy; therefore, their routine use outside of a clinical trial should be discouraged. It is also important to note that although the gut contains the largest and most well-studied microbiome, other microbiota communities within the body may also have cancer-modulating effects. For example, the lung and urinary tract microbiomes have potential roles in the development and progression of lung and urinary tract cancers, respectively.16,17
With the advent of new immunotherapy drugs, it becomes increasingly important to make them functional for as many patients with cancer as possible. Closely investigating the gut microbiome and its mechanisms will be beneficial in understanding different aspects of cancer treatment, especially checkpoint inhibitors, and in broadening our understanding of the immune system. With further research, it will be possible to create the most effective treatment combination of gut microbiota and immunotherapy to maximize patient outcomes.
Current Clinical Status
This research is still in the early stages. However, clinical trials are currently in progress with the aim of better understanding the composition of the gut microbiome and its role in the development of cancer and response to cancer treatments, particularly immunotherapies. Findings from these trials may inform additional studies aimed at modifying the gut microbiome to maximize its synergy with cancer immunotherapy. Results from such research could significantly impact patient care in the not-too-distant future.
References:
- Candeias SM, Gaipl US. The immune system in cancer prevention, development and therapy.Anticancer Agents Med Chem. 2016;16(1):101-107.
- Janeway CA, Travers P, Walport M, Shlomchik J.Immunobiology, 5th ed. 2001. New York: Garland Science. ISBN-10: 0-8153-3642-X.
- Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017;66(5):551-564. doi: 10.1007/s00262-017-1954-6.
- Kaiser J. Gut microbes shape response to cancer immunotherapy. Science. 2017;3;358(6363):573. doi: 10.1126/science.358.6363.573.
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J. 2006;26:154-158.
- McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77-e91. doi: 10.1016/S1470-2045(18)30952-30955.
- Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800-812. doi: 10.1038/nrc3610.
- Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330(6005):831-835. doi: 10.1126/science.1191175.
- Gori S, Inno A, Belluomini L, Bocus P, Bisoffi Z, Russo A, Arcaro G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy.Crit Rev Oncol Hematol.2019;143:139-147. doi: 10.1016/j.critrevonc.2019.09.003.
- Hampton T. Gut microbes may shape response to cancer immunotherapy. JAMA. 2018;319(5):430-431. doi: 10.1001/jama.2017.12857.
- Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018 Jun 1;29(6):1437-1444. doi: 10.1093/annonc/mdy103.
- Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97-103. doi: 10.1126/science.aan4236.
- Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084-1089. doi: 10.1126/science.aac4255.
- Suez J, Zmora N, Zilberman-Schapira G, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. 2018;174(6):1406-1423.e16. doi: 10.1016/j.cell.2018.08.047.
- Mao Q, Jiang F, Yin R, et al. Interplay between the lung microbiome and lung cancer. Cancer Lett. 2018;415:40-48. doi: 10.1016/j.canlet.2017.11.036.
- Aragón IM, Herrera-Imbroda B, Queipo-Ortuño M, et al. The urinary tract microbiome in health and disease. Eur Urol Focus. 2018;4(1):128-138. doi: 10.1016/j.euf.2016.11.001.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More