ESMO Issues Practice Guidelines Update for Treatment of Bladder Cancer
Several novel therapeutics have gained approval from the European Medicines Agency for the treatment of patients with bladder cancer in recent years, prompting the European Society for Medical Oncology to issue an eUpdate to these guidelines in August of 2019.
Thomas Powles, MD, MBBS
Several novel therapeutics have gained approval from the European Medicines Agency (EMA) for the treatment of patients with bladder cancer in recent years, prompting the European Society for Medical Oncology (ESMO) to issue an eUpdate to these guidelines in August of 2019.1
In 2014, ESMO issued guidelines for the management of various stages of bladder cancer treatment.2The update addressed the use of new therapeutics in platinum-refractory urothelial cancer and in patients with PD-L1positive urothelial cancer who are ineligible for cisplatin-based chemotherapy.1The eUpdate provides evidence-level support for each recommendation.
“The eUpdated urothelial/bladder cancer guidelines incorporate immune and targeted therapy into the disease pathway for the first time,” Thomas Powles, MD, MBBS, professor of genitourinary oncology and lead for solid tumor research at Barts Cancer Institute, director of Barts Cancer Centre, and an author on the eUpdate, toldTargeted Therapies in Oncologyin an interview. “[The eUpdate] recommend[s] immune therapy, particularly pembrolizumab [Keytruda], as the optimal treatment in platinum-refractory, metastatic disease. Other immune checkpoint inhibitors [ICIs] can be given with less-strong evidence. First-line pembrolizumab or atezolizumab [Tecentriq] can also be given in first-line metastatic PD-L1positive cisplatin-ineligible disease,” Powles said.
EMA and FDA Approvals in Urothelial Cancer
In 2017, the ICIs pembrolizumab and nivolumab (Opdivo) were approved for the treatment of urothelial cancer by the EMA.3,4Pembrolizumab was also approved for use by the FDA in 2017.5
In 2017, atezolizumab was approved for the treatment of advanced or metastatic urothelial cancer in the European Union, having been approved for use in the United States in 2016.6,7 In addition, the antibodydrug conjugate enfortumab vedotin received FDA Breakthrough Therapy Designation in March 2018.
Treating Platinum-Refractory Disease With ICIs
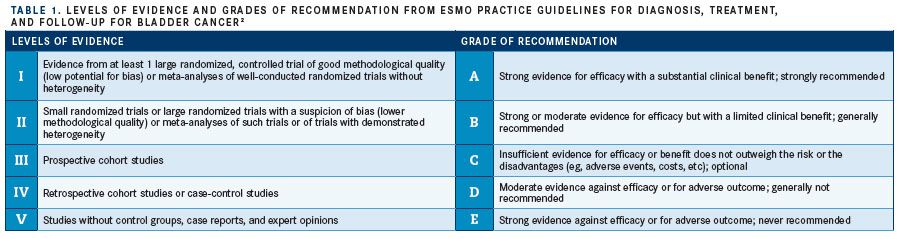
The ESMO eUpdate to the use of ICIs for management of platinum-refractory urothelial cancer supersede the 2014 guideline recommendations that indicated the use of the third-generation vinca alkaloid vinflunine (Javlor) in this setting. The agent bore IB-level evidence, as it is the only such therapeutic approved for this particular use (TABLE 1).2
The 2019 update recommends the use of therapeutic antibodies that target and block the PD-1 receptor and associated ligands, which are expressed in tumor cells and prevent T-cell activity in the immune response, based on clinical trial evidence from KEYNOTE-045 and IMvigor211.1,8,9The eUpdate also indicates that biomarkers should not be used in the selection of platinum-refractory patients for these treatments.
Pembrolizumab received the highest level of evidence based upon data showing it to be the only PD-1targeting antibody to have improved overall survival (OS) significantly in patients with urothelial cancer in a randomized phase III trial.1,8Patients treated with pembrolizumab experienced a median OS of 10.3 months compared with 7.4 months in the KEYNOTE-045 trial which examined both therapies in those with advanced, refractory urothelial cancer that recurred or progressed after platinum-based chemotherapy (HR, 0.73; 95% CI, 0.59-0.91;P= .002).8
Consideration for Other ICIs
The eUpdate assigned pembrolizumab IA level of evidence in patients with urothelial cancer refractory to platinum chemotherapy, with other ICI antibodies proposed for consideration based on lower levels of evidence.1The PD-L1targeting monoclonal antibody, atezolizumab, and the PD-1–targeting monoclonal antibody nivolumab have been assigned level IIB and IIIB evidence, respectively, based on available clinical-trial data. PD-L1–targeting agents avelumab (Bavencio) and durvalumab (Imfinzi) were recommended with lower levels of supporting evidence, and neither is approved by the EMA in this setting.1
In the multicenter, open-label, randomized, controlled phase III IMvigor211 trial, 931 patients with advanced urothelial cancer who progressed on platinum-based chemotherapy were randomized to atezolizumab or physician’s choice of chemotherapy, with a primary end point of OS.9
The study found the OS rate at 12 months to be 39.2% (95% CI, 35%-44%) in the atezolizumab group versus 32.4% (95% CI, 28%-37%) in the chemotherapy group (HR, 0.85; 95% CI, 0.73- 0.99). Although the intervention did not lead to statistically significant improvements in OS over chemotherapy, the atezolizumab group had fewer grade 3/4 adverse events (AEs).9
The single-arm, open-label phase IIIb SAUL study focused on atezolizumab’s safety profile and included patients with advanced urothelial cancer, 98% of whom received previous platinum-based therapy. This trial included patients were ineligible for the IMvigor211 trial. Secondary end points included efficacy evaluations of OS, progression-free survival (PFS), and overall response rate (ORR).10
Atezolizumab was administered intravenously every 3 weeks until loss of clinical benefit or unacceptable toxicity were observed, investigator or patient decision to discontinue, or death. Discontinuations due to disease progression or AEs occurred in 66% and 8%, respectively. Grade ≥3 treatment-related AEs (TRAEs) included fatigue, asthenia, colitis, and hypertension in 1% of patients each.10
The OS rate at 6 months was 60% (95% CI, 57.0%-63.0%) and at 1 year, 41% (95% CI, 38%-44%). The median PFS was 2.2 months (95% CI, 2.1- 2.4) and 2.8 months (95% CI, 2.4-3.4) according to modified RECIST. ORR by RECIST was 13%, including 3% complete responses (CRs).10
Because of atezolizumab’s weaker performance in clinical trial’s, ESMO’s eUpdate recommends the agent with this lower level of evidence compared with pembrolizumab.
PD-L1 BiomarkerPositive, Cisplatin-Ineligible Disease
Nivolumab was investigated in the multicenter, single-arm, phase II CheckMate 275 trial that enrolled patients with advanced or metastatic urothelial cancer with varying tumor PD-L1 expression and a primary endpoint of ORR. The reported ORR was 19.6% (95% CI, 15%-25%) in all treated patients, with response rates of 28.4%, 23.8%, and 16.1% for patients with PD-L1 expression ≥5%, ≥1%, and <1%, respectively.11The 2014 guidelines recommended carboplatin-based therapy, gemcitabine, or a taxane as single agents in the frontline for patients with urothelial cancer who are ineligibile for cisplatin. The methotrexate/carboplatin/vinblastine or carboplatin/gemcitabine regimens were also recommended as considerations with level IA evidence.2
The eUpdate maintains its recommendation of carboplatin-based chemotherapy as a therapeutic for these patients and adds pembrolizumab and atezolizumab as considerations for use. The update follows the EMA and FDA recommendations that ICIs only be used in patients who are biomarker positive or have tumor PD-L1 expression >5%, with level IIIB evidence for both agents.1
The use of pembrolizumab is supported by data from the phase II KEYNOTE-052 trial that examined the agent in patients with urothelial cancer who were ineligible for cisplatin-based therapy. Pembrolizumab demonstrated a 24% (95% CI, 20%-29%) response rate and a 47% (95% CI, 42%-52%) disease control rate.12
Patients With Platinum-Refractory Urothelial Cancer
Similarly, atezolizumab was investigated in a multicenter, single-arm, phase II study of patients with locally advanced or metastatic disease who were ineligible for cisplatin-based therapy that resulted in a response rate of 23% (95% CI, 16%-31%) and a median OS of 15.9 months (95% CI, 10.4-not estimable). The 1-year landmark survival rate was 57% (95% CI, 48%-66%) in all patients.13A third update makes recommendations for the treatment of patients with platinum- and ICI-refractory urothelial cancer who have aberrations inFGFR, includingFGFR2orFGFR3alterations. ESMO now recommends the use of the FGFR tyrosine kinase inhibitor erdafitinib (Balversa) or the antibodydrug conjugate enfortumab vedotin with level IIIB evidence in this patient population.1
Enfortumab vedotin delivered intravenously targets Nectin-4, which has been shown to be highly expressed in urothelial cancer. Although not approved for use by the EMA or the FDA, the agent was investigated in a single-arm, phase II study, and the results showed an ORR of 44% (95% CI 35%-53%) in patients with advanced or metastatic urothelial cancer previously treated with platinum therapy and ICIs. CRs were observed in 12% and partial responses (PRs) in 32%; stable disease was experienced by 28%.14
Median PFS and OS were 5.8 months (95% CI, 4.9-7.5) and 11.7 months (95% CI, 9.1-not reached [NR]), respectively. The most commonly reported any-grade TRAEs included fatigue (50%), alopecia (49%), decreased appetite (44%), dysgeusia (40%), peripheral sensory neuropathy (40%), and nausea (39%).
Also not approved for use by the EMA, erdafitinib was granted accelerated approval by the FDA in 2019.15 In an open-label, phase II trial of patients with locally advanced unresectable or metastatic urothelial cancer andFGFRalterations, randomization assigned patients to either intermittent or continual erdafitinib dosage, with ORR as the primary endpoint.16

The ORR was 40% (95% CI, 31%-50%), comprising 3% of patients with CRs and 37% with PRs, and the investigators found that the response rates were similar regardless of chemotherapy history. The secondary endpoint of OS was found to be 13.8 months (95% CI, 9.8-NR), and the OS rate at 1 year was 55% (95% CI, 43%-66%).16
The strongest recommendation in the eUpdate to the 2014 ESMO guidelines is for the use of pembrolizumab in platinum-refractory urothelial cancer, with lower-level evidence in other agents for the treatment of this patient population; those being treated in the first line with cisplatin-ineligible, PD-L1positive tumors; and those with platinum- and ICI-refractory disease (TABLE 2).1Although clinical trials continue to investigate erdafitinib and enfortumab vedotin, they have been recommended by the update despite not being approved by the EMA.
References:
- eUpdate: bladder cancer treatment tecommendations. European Society for Medical Oncology website. bit.ly/2mle4JD. Published August 22, 2019. Accessed September 23, 2019.
- Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A; ESMO Guidelines Working Group. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up.Ann Oncol. 2014;25(suppl 3):iii40-iii48. doi: 10.1093/annonc/mdu223.
- European Commission approves Merck’s Keytruda (pembrolizumab) for the treatment of certain patients with locally advanced or metastatic urothelial carcinoma, a type of bladder cancer [news release]. Kenilworth, NJ: Merck; September 5, 2017. bit.ly/2mmgkjQ. Accessed September 30, 2019.
- European Commission approves Bristol-Myers Squibb’s Opdivo (nivolumab) for previously treated locally advanced unresectable or metastatic urothelial carcinoma in adults after failure of prior platinum-containing therapy [news release]. Princeton, NJ: Bristol-Myers Squibb; June 2, 2017. bit.ly/2tu5Zlf. Accessed September 30, 2019.
- Pembrolizumab (Keytruda): advanced or metastatic urothelial carcinoma. FDA website. bit.ly/2oIgNOd. Published May 18, 2017. Accessed September 30, 2019.
- Roche receives EU approval of Tecentriq (atezolizumab) in a specific type of metastatic lung cancer and two types of metastatic bladder cancer [news release]. Basel, Switzerland: Roche; September 22, 2017. bit.ly/2nSn7lL. Accessed September 30, 2019.
- Atezolizumab for urothelial carcinoma. FDA website. bit.ly/2nZDY6o. Published May 18, 2016. Accessed September 30, 2019.
- Bellmunt J, de Wit R, Vaughn DJ, et al; KEYNOTE-045 Investigators. Pembrolizumab as second-line therapy for advanced urothelial carcinoma.N Engl J Med. 2017;376(11):1015-1026. doi: 10.1056/NEJMoa1613683.
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial.Lancet. 2018;391(10122):748-757. doi: 10.1016/S0140-6736(17)33297-X.
- Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract.Eur Urol. 2019;76(1):73-81. doi: 10.1016/j.eururo.2019.03.015.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial.Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7.
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study.Lancet Oncol. 2017;18(11):1483-1492. doi: 10.1016/S1470-2045(17)30616-2.
- Balar AV, Galsky MD, Rosenberg JE, et al; IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial.Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2.
- Petrylak DP, Balar AV, O’Donnell PH, et al. EV-201: results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and immune checkpoint inhibitors.J Clin Oncol. 2019;37(suppl 18;abstr LBA4505). ascopubs.org/doi/abs/10.1200/JCO.2019.37.18_suppl.LBA4505?af=R.
- FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. FDA website. bit.ly/301B7bB. Published April 12, 2019. Accessed September 30, 2019.
- Loriot Y, Necchi A, Park SH, et al; BLC2001 Study Group. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma.N Engl J Med. 2019;381(4):338-348. doi: 10.1056/NEJMoa1817323.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More