Immune Checkpoint Inhibitors Approach Frontline Setting in Head and Neck Squamous Cell Cancer
Immune checkpoint inhibitors as monotherapy and in combinations regimens are producing promising efficacy data in metastatic head and neck cancers, depite struggles in finding the right treatment settings and patient population subsets.
Barbara Burtness, MD
Immune checkpoint inhibitors as monotherapy and in combinations regimens are producing promising efficacy data in metastatic head and neck cancers, depite struggles in finding the right treatment settings and patient population subsets.
The overall incidence of head and neck squamous cell cancer (HNSCC) is declining in North America, particularly for nonoropharyngeal squamous cell carcinoma, among both men and women.1However, the prognosis for recurrent or metastatic HNSCC remains poor, with a median survival of less than 1 year.2Research into improving survival remains challenging, yet there has been some headway in recent year. This could be, in part, due to the rising incidence of human papilloma virus (HPV)-related tumors that are inderstood to be more sensitive to chemotherapy and radiation therapy than non-HPVrelated disease.3
Nabil Saba, MD
There are several ongoing trials at different stages of accrual for HNSCC using immune checkpoint inhibitor combinations with chemotherapy or radiotherapy. Additionally, the variable success, or failure, observed in clinical trials in patients with advanced HNSCC achieving long-term disease control and some long-lasting remissions with single-agent immune checkpoint inhibitors are of particular interest.Long-term remissions have not been observed using traditional chemotherapy regimens in patients with recurrent or metastatic disease, said Nabil F. Saba, MD, professor of hematology and medical oncology and otolaryngology, Emory University School of Medicine, and director of the Winship Cancer Institute’s Head and Neck Medical Oncology Program, both in Atlanta, Georgia. “Achieving these results with a less toxic approach was inconceivable as recently as 5 or 6 years ago. I think we are essentially revolutionizing systemic therapy for [patients with] HNSCC.”
Prospective Frontline Indication for Pembrolizumab
In February 2019, the FDA accepted a new supplemental biologics license application for pembrolizumab as monotherapy or in combination with platinum and 5-fluorouracil (5-FU) chemotherapy for the frontline treatment of patients with recurrent or metastatic HNSCC.4
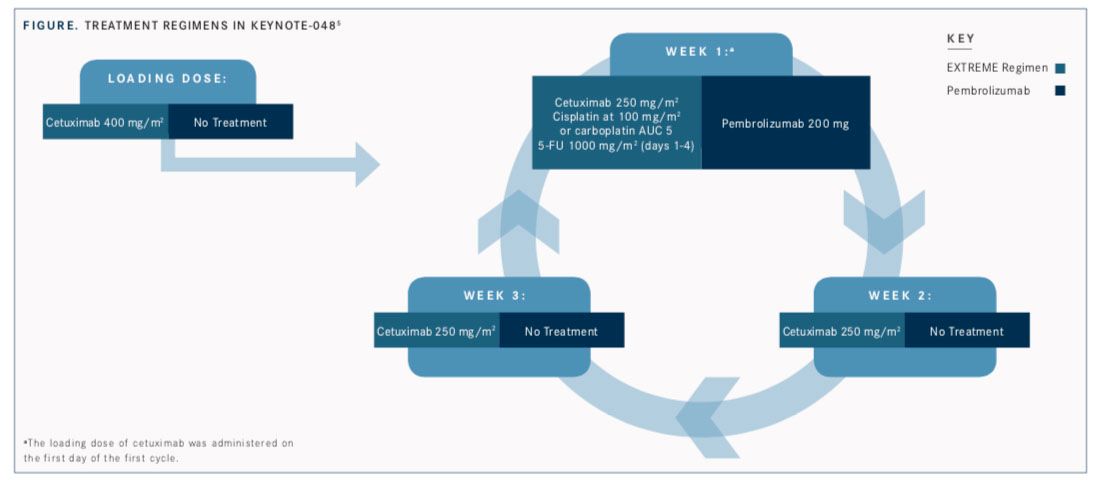
The application was based on results of the phase III KEYNOTE- 048 trial, which compared pembrolizumab (Keytruda) as a single agent or pembrolizumab in combination with chemotherapy with standard-of-care cetuximab (Erbitux) plus platinum chemotherapy and 5-FU (the EXTREME regimen;FIGURE). Interim findings were reported at the European Society of Medical Oncology (ESMO) 2018 Congress.5
Since pembrolizumab was approved as the first PD-1 inhibitor in 2014, it has received indications for an unprecedented 11 tumor types and was granted accelerated approval in August 2016 for the treatment of patients with recurrent or metastatic HNSCC with disease progression on or after platinum-containing chemotherapy.
Patients in KEYNOTE-048 had not received chemotherapy or systemic therapy for recurrent or metastatic squamous cell carcinoma of the oral cavity, the oropharynx, the larynx, or the hypopharynx. Although the PD-1 inhibitor did not provide an improvement in progression-free survival (PFS; HR, 0.92; P = .2) or objective response rate (ORR) in the overall population, monotherapy with pembrolizumab did improve overall survival (OS) and duration of response (DOR) in patients with a PD-L1 combined positive score (CPS) ≥1. For the total population, pembrolizumab was determined to be noninferior to the EXTREME regimen for OS.
“I think [KEYNOTE-048] is an extremely important study that is going to transform the way we take care of patients with head and neck cancer,” said Barbara Burtness, MD, professor of medicine (medical oncology), Disease Aligned Research Team leader for head and neck cancers, and codirector of the Developmental Therapeutics Program at Yale University School of Medicine in New Haven, Connecticut.
“The initial hypothesis was that pembrolizumab would be better than EXTREME in patients [with high PD-L1 expression]; that is, those who had a CPS ≥20,” Burtness explained in an interview withTargeted Therapies in Oncology. “The analysis of that arm showed that pembrolizumab is superior to the EXTREME regimen for patients who have CPS ≥20, that it is superior in terms of OS for patients who have CPS ≥1, and that it is noninferior for patients whether or not they have PD-L1 expression. And we are anticipating that a final analysis will be presented at a later meeting.”
Burtness noted that pembrolizumab was superior to the EXTREME regimen for OS survival in the CPS ≥20 population and that the hazard ratio for death for this patient population was 0.61, which is highly statistically significant. The median OS with pembrolizumab was 14.9 months versus 10.7 months in the control arm. “This happened despite the fact that pembrolizumab does not lead to as high a response rate and actually had a somewhat shorter PFS,” she added. The difference in PFS for this population was not statistically significant (HR, 0.99; P = .5).
The results for the CPS ≥1 population were not as dramatic. The median OS was 10.3 months with EXTREME versus 12.3 months with pem- brolizumab (HR, 0.78; P = .0086). In this group, more than 30% of the patients were alive at 24 months. For the pembrolizumab and chemother- apy combination, the hazard ratio was 0.77 for all participants, and 29% were alive at 24 months compared with 18% who received the EXTREME regimen, Burtness said. “I think there’s still more to be learned from this study in terms of what is the best treatment for patients with low PD-L1 expression and discerning when the addition of chemotherapy is going to make a big difference,” she said.
The safety profile of pembrolizumab monotherapy, which is consistent with that in previous studies, is less toxic compared with the EXTREME regimen, according to Burtness. And when pembrolizumab is combined with chemotherapy, the toxicity profiles are comparable.
Placing Durvalumab in the Right Setting
Investigators were optimistic about the use of durvalumab (Imfinzi), a human immunoglobulin G1 κ monoclonal antibody, in patients with recurrent or metastatic HNSCC and high PD-L1 expression (≥25%) who progressed on platinum-based chemotherapy following positive results from the phase II HAWK study (NCT02207530) presented at the ESMO 2017 Congress.6
The primary endpoint of ORR for patients receiving durvalumab was 16.2% (95% CI, 9.9%- 24.4%).7For all patients, the median PFS was 2.1 months (95% CI, 1.9-3.7) and OS was 7.1 months (95% CI, 4.9-9.9). There was a 12-month survival rate of 33.6% (95% CI, 24.8%-42.7%). Later studies were not as promising, however. The addition of durvalumab was fairly well tolerated, as seen in HAWK, and these results led to phase III studies of the agent with or without tremelimumab, a CTLA-4 inhibitor, in EAGLE (NCT02369874) and KESTREL (NCT02551159).
An update regarding the phase III, open-label EAGLE trial, which investigated the use of durvalumab alone and in combination with tremelimumab compared with standard chemotherapy yielded disappointing results, according to a press release issued in late 2018 by AstraZen- eca, the manufacturer of durvalumab.8However, the company remains committed to further study of durvalumab for other indications in head and neck cancer. The results of the EAGLE trial will be presented at an upcoming conference.
“[EAGLE] did not support a combination approach so far in advanced HNSCC,” Saba said. However, other trials, such as KESTREL, are in progress and may shed further light on the activity of combination therapies of PD-1/PD-L1 and CTLA-4 inhibitors, he added.
Sean Bohen, executive vice president of global medicines development and chief medical officer of AstraZeneca, was optimistic about the efficacy of durvalumab in other clinical trials. “We look forward to seeing the results of the phase III KESTREL trial of Imfinzi and tremelimumab in patients who have not received prior chemotherapy for recurrent or metastatic HNSCC in the first half of 2019,” he said in a press release.
The phase II, randomized, open-label CONDOR trial, whose findings were published inJAMA Oncologyin November 2018,9 also examined the PD-L1/ CTLA-4 inhibitor combination. The study compared durvalumab or tremelimumab monotherapy, or durvalumab plus tremelimumab, in patients with recurrent or metastatic HNSCC and low or no PD-L1 expression. The ORRs were 9.2% (95% CI, 3.46%- 19.02%), 1.6% (95% CI, 0.04%-8.53%), and 7.8% (95% CI, 3.78%-13.79%) in the durvalumab, tremelimumab, and combination arms, respectively, with no complete responses reported. Tumor shrinkage was experienced in 23.1% of patients in the durvalumab arm, 14.3% in the tremelimumab arm, and 31% in the combination arm. Median OS for patients in the combination cohort was 7.6 months (95% CI, 4.9- 10.6), 6 months (95% CI, 4.0-11.3) for durvalumab alone, and 5.5 months (95% CI, 3.9-7.0) for tremelimumab alone.
Among patients who received the combination therapy, 15.8% experienced grade 3/4 treatment-related adverse events (AEs) compared with 12.3% on durvalumab monotherapy and 16.9% with tremelimumab monotherapy. Grade 3/4 immune-mediated AEs were noted only in the combination arm, affecting 6.0% of patients.
“In the phase II CONDOR trial, durvalumab... showed an ORR consistent with other single-agent PD-1/PD-L1 inhibitors in second-line settings for head and neck cancer,” said Lillian Siu, MD, lead investigator on the study, senior medical oncologist at Princess Margaret Cancer Centre, and a professor of medicine at the University of Toronto, Canada, in a press release. “Our results add to the body of evidence that this immune checkpoint inhibitor is tolerable and has demonstrated encouraging clinical activity across a range of tumors, including in heavily pretreated recurrent or metastatic head and neck cancer.”
Promising Results for Motolimod in HPV+ HNSCC
Motolimod, a small molecule agonist of toll-like receptor 8, has been found in early trials to induce positive immune responses in HNSCC and was examined in the phase II Active8 trial (NCT01836029) in patients with recurrent or metastatic HNSCC.10Motolimod added to the EXTREME regimen did not improve PFS (6.1 vs 5.9; HR, 0.99; P = .47) or OS (13.5 vs 11.3; HR, 0.95; P = .4) over placebo. However, there was a significant improvement in survival outcomes among patients with HPV-related HNSCC.
In a prespecified subgroup analysis of patients with HPV-positive disease, patients receiving motolimod had a median PFS of 7.8 months versus 5.9 months for placebo (HR, 0.58; P = .046). Median OS was also improved, with the motolimod cohort at 15.2 months versus 12.6 months (HR, 0.41; P = .03) for placebo.
There were also positive data among patients who experienced injection-related site reactions. The motolimod arm had a median PFS of 7.1 months versus 5.9 months in the placebo group (HR, 0.69; P = .06). Median OS was 18.7 months for the motolimod group versus 12.6 months in the placebo group (HR, 0.56; P = .02).
These results raise the question of whether this or similar agents could have a benefit in a subset of patients with HPV-positive HNSCC or injection-related site reactions, said Saba.
Nivolumab Clinical Trials in the Frontline Setting
Nivolumab (Opdivo), another PD-1 immune checkpoint inhibitor, is approved by the FDA as monotherapy for the treatment of recurrent or metastatic HNSCC that progressed after platinum-based chemotherapy.11This approval was based on data from the CheckMate 141 trial (NCT02105636) that showed that patients with disease progression after platinum-based chemotherapy experienced significantly longer OS (7.5 months) with nivolumab than they did with treatment that included standard therapy (5.1 months; HR, 0.70; 95% CI, 0.53-0.92; P = .0101). There were no statistically significant differences between the 2 arms for PFS (HR, 0.89; 95% CI, 0.70, 1.13) or ORR (13.3% vs. 5.8%) for nivolumab and investigator’s choice, respectively.
The agent is now being examined for the first-line treatment of patients with recurrent or metastatic HNSCC in combination with ipilimumab (Yervoy) in the ongoing phase III CheckMate 651 trial (NCT02741570). The 2-arm, open-label trial is comparing the combination with the EXTREME regimen, with coprimary endpoints of OS and PFS in patients with PD-L1 expressing tumors.
Radiation Therapy
Several studies have been looking at stereotactic body radiation therapy (SBRT) as additional therapy for patients with metastatic head and neck carcinoma who are receiving systemic immunotherapy agents in hopes that the dual therapies will act synergistically to control local and systemic disease.
A new phase I/II single-arm study (NCT03283605) is treating patients with combination durvalumab plus tremelimumab followed by durvalumab alone until disease progression, unacceptable toxicity, or patient withdrawal. Between the second and third cycles of the combination therapy, the patients receive SBRT.
A multi-institution trial in collaboration with Emory University and the Cleveland Clinic is currently adding nivolumab to radiation therapy in patients with recurrent or second primary HNSCC. These patients have had exposure to prior radiation therapy and therefore are at par- ticularly increased risk for toxicity and treatment failure, said Saba. Therefore, it makes sense to rely on the less toxic and possibly more effective immune checkpoint inhibitors rather than chemotherapy in this setting, he added. The trial (NCT03521570) is currently accepting patients.
Moving Forward
Not all trials of immunotherapy treatment in HNSCC have provided the positive findings that investigators had hoped for, but they do remain hopeful, particularly because of the lower toxicity profiles of immunotherapy agents compared with standard-of-care regimens such as EXTREME. Adding to this are the positive findings associated with pembrolizumab.
“I hope the FDA is going to approve pembrolizumab for first-line use because I think we have evidence that this is a way to help our patients live longer,” said Burtness. “I think we need to see what the patients [with low PD-L1 expression] do and whether or not pembrolizumab is better saved for later-line therapy or ought to be given early in these patients.
“There is a slightly higher incidence of early progression on the pembrolizumab monotherapy arm, so if there were biomarkers that could help us predict those patients, that would be useful,” Burtness said, opening the door for future investigations into the efficacy of immune checkpoint inhibitors in certain patient subsets. “Early progression is not predicted simply by being PD-L1 negative, because you can see early progression in PD-L1expressing cancers as well,” she added.
“In ECOG-ACRIN, we have a trial just about to activate for immediate-risk HPV-associated cancer [NCT03811015], where patients are going to be given definitive platinum radiation and then, afterward, get either nivolumab or observation,” Burtness said.
Much more remains to be done to extend survival for patients with HNSCC. Investigators are continuing to look at integrating more complex immune profiling, as well as interferon response gene signatures, tumor mutational burden, and other predictive characteristics within the patient’s tumors, Burtness concluded.
References:
- Fakhry C, Krapcho M, Eisele DW, D’Souza G. Head and neck squamous cell cancers in the United States are rare and the risk now is higher among white individuals compared with black individuals. Cancer. 2018;124(10):2125-2133. doi: 10.1002/cncr.31322.
- Brockstein BD, Vokes EE. Treatment of metastatic and recurrent head and neck cancer. UpToDate website. bit.ly/2UTOw2t. Updated March 1, 2019. Accessed March 1, 2019.
- Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994- 1001.doi:10.1634/theoncologist.2009-0289.
- FDA grants priority review to Merck’s supplemental biologics license application for Keytruda (pembrolizumab) for the first-line treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) [news release]. Kenilworth, NJ: Merck; February 11, 2019. bit.ly/2MXWNPw . Accessed March 1, 2019.
- Burtness B, Harrington KJ, Greil R, et al. KEYNOTE-048: phase 3 study of first- line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC). Presented at: European Society of Medical Oncology 2018 Congress; October 19-23, 2018; Munich, Germany. Abstract LBA8_PR. bit.ly/2HY1hp9.
- Zandberg D, Algazi A, Jimeno A, et al. Durvalumab for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): preliminary results from a single-arm, phase 2 study. Presented at: European Society of Medical Oncology 2018 Congress; October 19-23, 2018; Munich, Germany. Abstract 10420. bit.ly/2FtyJ3J.
- ESMO 2017: durvalumab shows promising clinical benefit in recurrent/ metastatic head and neck squamous cell carcinoma. European Society of Medical Oncology website. bit.ly/2TYNrtw. Published September 11, 2017. Accessed March 7, 2019.
- Update on the phase III EAGLE trial of Imfinzi and tremelimumab in ad- vanced head and neck cancer [news release]. Gaithersburg, MD: AstraZeneca, Medimmune; December 7, 2018. bit.ly/2HqgWhZ. Accessed March 1, 2019.
- Siu LL, Even C, Mesía R, et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial [published online November 1, 2018]. JAMA Oncol. doi: 10.1001/jamaoncol.2018.4628.
- Ferris RL, Saba NF, Gitlitz BJ, et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squa- mous cell carcinoma of the head and neck: the Active8 randomized clinical trial. JAMA Oncol. 2018;4(11):1583-1588. doi:10.1001/jamaoncol.2018.1888.
- Nivolumab (Opdivo) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2014.
