Hutson Reviews Multiple Combination Trials for Patients With RCC
During a Targeted Oncology case-based roundtable event, Thomas Hutson, DO, PharmaD, discussed immunotherapy and tyrosine kinase inhibitor combinations for frontline therapy of renal cell carcinoma.
Thomas Hutson, DO, PharmD
Director, Urologic Oncology Program
Cochair, Urologic Cancer Research and Treatment Center
Baylor University Medical Center
Physician, Texas Oncology-Baylor Charles A. Sammons Cancer Center
Dallas, TX

Targeted OncologyTM: What are the recommendations and data behind the options for this patient?
HUTSON: In the National Comprehensive Cancer Network [NCCN] guidelines, there is a separation of first-line therapy for clear cell histology based on favorable-, poor-, or intermediate-risk groups. This patient meets the criteria for [the] intermediate-risk category based on the IMDC [International Metastatic RCC Database Consortium] risk calculator. And all the preferred regimens for this group, except single-agent cabozantinib [Cabometyx], have a category 1 recommendation.1
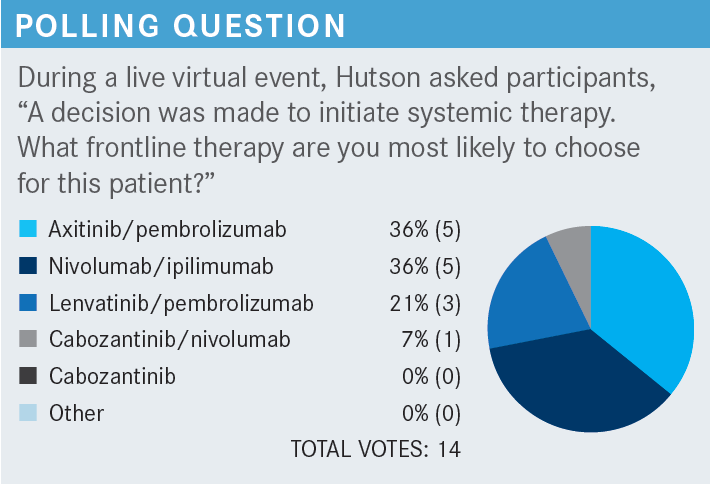
Any of the regimens would be appropriate to choose, but there are nuances between them. The polling suggests axitinib [Inlyta] plus pembrolizumab [Keytruda]—being one of the first regimens to be in the market—has been a dominant TKI [tyrosine kinase inhibitor] and immunotherapy [IO] combination. Ipilimumab [Yervoy] plus nivolumab [Opdivo], the immune-based regimen, is the alternative. I think, even right now, the market share is between these 2 combinations.
The various primary therapeutic options for the frontline setting [have study data]. For ipilimumab plus nivolumab, it is the CheckMate 214 trial [NCT02231749], which was the first one published, so it has the longest follow-up.2 The other 3 TKI plus IO trials are: KEYNOTE-426 [NCT02853331] for axitinib plus pembrolizumab, CheckMate 9ER [NCT03141177] for cabozantinib plus nivolumab, and the CLEAR study [NCT02811861] for lenvatinib [Lenvima] plus pembrolizumab. There are various efficacy markers comparing [all the combinations].
How do these trials compare for patients with RCC?
The CheckMate 214 trial has a greater than 5-year follow-up, KEYNOTE 426 has a 42-month follow-up, CheckMate 9ER [has 23.5 months], and [CLEAR has 33.7 months] follow-up.2-5 This has given pause to people looking at the data with some feeling that it may or may not hold up over time.
Ipilimumab plus nivolumab was predominantly studied in the intermediate- and poor-risk groups. Therefore, the bulk of patients evaluated were in these groups. Those in the favorable-risk group who found their way into therapy tended to not have the benefits that were seen in the intermediate- and poor-risk groups, but again, it was a small number of patients.2
The other 3 IO/TKI trials enrolled patients irrespective of their risk group. The CheckMate 9ER trial had built into its design a cap on the number of patients in the favorable-risk group to approximately 20%, and that’s why it had the lowest number from this group.4 But the other trials allowed whoever came in the door, and the breakdown is not dissimilar to what we would see in clinical practice. Normally we would expect to see approximately 20% favorable risk, approximately 60% intermediate risk, and approximately 20% poor risk.
Based on that alone, not all these trials were the same. There were slight differences between the populations that are enrolled, and to be honest and fair, we don’t know how significant these differences are in affecting the outcome. These are questions that are brought up when one does cross-trial comparisons.
What was the efficacy observed in the RCC trials?
For the median overall survival [mOS], we have the most mature data with ipilimumab plus nivolumab at [55.7 months (HR, 0.72; 95% CI, 0.62-0.85)]. This is a good way to standardize the data and look at the benefit. This translates to a 28% risk reduction in death, in favor of ipilimumab plus nivolumab. The hazard ratios of the other agents are within a few points of each other. The benefit is very similar among the agents, with none necessarily standing out more than the other.
The landmark analysis [of OS at] 12 months and 24 months across the trials are all within 10 percentage points of each other.2-5
Hazard ratios of OS are within the same range, but there is a difference [based on] whether it’s intermediate-, poor-risk, or all-comers. [The hazard ratios for the CheckMate 9ER and CLEAR studies for the intermediate-risk groups are 0.74 and 0.72, respectively. For the poor-risk groups, they are 0.45 and 0.39, respectively].4,5
[Looking at the data], the hazard ratios for PFS [progression-free survival] are probably where things differentiate the most, with the least impact on PFS [from] ipilimumab plus nivolumab at 0.86 [95% CI, 0.73-1.01], where the IO plus TKI agents seem to have much more impact on PFS, with a regimen like lenvatinib plus pembrolizumab having a profound benefit in the PFS, with a hazard ratio of 0.39 [95% CI, 0.32-0.49].2,5 For the intermediate- and poor-risk groups, the IO plus TKI agents seem to do better there than what we see with ipilimumab plus nivolumab.
When we were developing immune therapies, [the right end point] wasn’t clear because there was a concern that looking at PFS, which has been the end point for the oral TKIs, may not be an appropriate measure of their benefit. We recognize there may not be significant differences in PFS because the immune therapy may take a while to work, and the best indicators of benefit [are] things like OS. The data somewhat support that because the immune therapies seem to have less impact on PFS.
How did patients in these trials respond to therapy?
For the traditional objective response rates [ORRs] across the board, ipilimumab plus nivolumab had a greater response rate compared with its control arm, which was sunitinib [Sutent]. But with the IO plus TKI agents, the response rates are greater. More people responded to the IO plus TKI regimen. The CR [complete response] rate, which is something we’ve never had before in kidney cancer, has now become a differentiator, and there’s a 12% CR rate with the ipilimumab plus nivolumab. For the IO plus TKI agents, the one that stands out is lenvatinib plus pembrolizumab, with a 16% CR rate.2,5
We know CR and ORR are dependent on the length of follow-up, meaning patients can develop responses over time. The [most mature] data are [from the combination of] ipilimumab plus nivolumab, so we feel more comfortable with those results than what we see with the CLEAR trial results, which is at a relatively early point to check for benefit. So the question that has come up in the debates between the 2 IO agents or the IO plus TKI agents has to do with the durability of response. With 5-year data, we are comfortable with the outcomes of ipilimumab plus nivolumab, but with the IO plus TKI agents not having as long of a follow-up, the question about whether the responses are indeed as durable is raised. For the intermediate- and poor-risk groups, the IO plus TKI agents are doing better.
So a high-level summary of the data would be that the IO agents in the intermediate- and poor-risk group, which is where the pivotal trial was conducted, show a benefit in patients out at 5 years. But the number of patients responding to that therapy is less than with the IO plus TKI combination. There’s [an approximately] 20% to 30% difference in response. The IO plus TKI regimens are going to produce a greater likelihood of having some type of objective tumor response, and you don’t have that with the 2 IO agents. What we have with them, although it works in less people, is a group of patients who are having durable benefit. We’re not sure whether the durability seen with the IO plus TKI agents will pan out. Everyone is focused in on the tail of the curves with these therapies.2-5 The most recent FDA approvals were in January 2021 for cabozantinib plus nivolumab, and then in August 2021 for lenvatinib plus pembrolizumab.6,7

What are the specific data for other options the patient could have received?
The phase 3 CLEAR study had the combination of lenvatinib plus pembrolizumab vs lenvatinib plus everolimus [Afinitor] vs sunitinib. This was an international trial. One needed to have advanced clear cell RCC and good organ function. Patients were stratified as to whether they had favorable-, intermediate-, or poor-risk disease—all of whom were enrolled. The statistics were set up so it was the lenvatinib plus pembrolizumab arm compared with sunitinib or the lenvatinib plus everolimus arm compared with sunitinib, and not the lenvatinib arms compared with each other.5
The lenvatinib dose was 20 mg daily, which was the dose from its clinical development, combined with pembrolizumab at 200 mg [intravenous] every 3 weeks; the traditional lenvatinib plus everolimus daily dosing at 18 mg and 5 mg, respectively; and the sunitinib was the traditional 50-mg, 4-weeks on, 2-weeks-off dose. The primary end point was PFS, and secondary end points were OS, ORR, safety, and quality of life. They looked at duration of response and biomarkers [as key exploratory end points].
What was the efficacy in the CLEAR trial?
[For our discussion], we’re most interested in [the lenvatinib plus pembrolizumab arm vs sunitinib arm]. There were obvious separations in their PFS curves, which was the primary end point. At a median follow-up of 26.6 months, there was a 14.7-month difference in PFS, in favor of the lenvatinib plus pembrolizumab over the sunitinib [HR, 0.39; 95% CI, 0.32-0.49; P < .001].
That’s a profound difference in benefit. As an aside, lenvatinib plus everolimus seemed to do very well compared with sunitinib, but again, the focus for us is on lenvatinib plus pembrolizumab.
Across the board, against potential confounders we stratified against, there was benefit for all subgroups in the lenvatinib plus pembrolizumab arm compared with the sunitinib arm, regardless of age, sex, region, [Memorial Sloan Kettering Cancer Center] risk group, IMDC risk group, performance status, sites of disease, and PD-L1 status.
The prespecified analysis of some of the adverse prognostic factors, which are things we think may affect outcome, such as bone metastases that can be difficult to treat, liver metastases, and sarcomatoid histology, did not influence the benefit of lenvatinib plus pembrolizumab over sunitinib. Neither did the nephrectomy status or PD-L1 status.8
The OS is something that is not mature yet on the initial analysis and will need to be read out for a longer time to make it comparable with ipilimumab plus nivolumab. The hazard ratio of 0.66 [95% CI, 0.49-0.88; P = .005] is in favor of lenvatinib plus pembrolizumab, not sunitinib, but that hazard ratio is in the same range with the other agents. Despite the excitement about PFS that we saw, [as far as OS is concerned], lenvatinib plus pembrolizumab seems to be like the other regimens.5
For a 33.7-month follow-up, the hazard ratio for mOS was 0.72 [95% CI, 0.55-0.93].9 With a longer follow-up, we may see the hazard ratio increase, and that is the concern with these trials, because the hazard ratios tend to creep up and we have less benefit with further follow-up. That’s another caveat when one compares across the trials.
For the tumor ORRs, the lenvatinib plus pembrolizumab arm had a shockingly high CR rate of 16.1% independently reviewed vs 4.2% for sunitinib, and the stable disease rate was 19.2% with lenvatinib plus pembrolizumab vs 38.1% with sunitinib. When you add up everything, it is in the 90% range of clinical benefit rate, which is very attractive.5 That means the first scan you do in the patient who you started on lenvatinib plus pembrolizumab has a high likelihood of at least showing stable disease. In my opinion, that matters. It’s very difficult to go to a patient [after the first therapy and scan] and say it isn’t working. Having a therapy that, in general, can produce at least stable disease is profound. There is a 70% chance of having some degree of tumor shrinkage with lenvatinib plus pembrolizumab. So again, two-thirds of patients are having benefit, which is great.
[Again], the median duration of response is low because it’s one of the newest trials, and we want to be able to confirm this benefit is maintained. But 74.3% of patients at least with a CR were maintained at 36 months.5 You are losing some patients as time goes on, and the concern with responses is that, with longer follow-up, the data won’t look as good. The lenvatinib plus pembrolizumab is going to have a greater duration of treatment response, as we’d expect, because of its PFS benefit.
Please discuss the safety profile for lenvatinib/pembrolizumab.
Each regimen was equally toxic, with over 80% of patients having at least grade 3 or greater toxicity. That shouldn’t be a surprise because we know, for lenvatinib—at least when it’s combined with everolimus—60% to 70% of patients require dose reductions. So we know there are adverse events [AEs] with this regimen, and they just seem to be slightly more than they are with sunitinib. What’s most important, though, is [whether] you can manage those AEs in the clinic so patients can stay on therapy. Despite having high levels of AEs, they are manageable in the clinic. So it’s only 13% of patients who [must] discontinue therapy permanently, and that speaks to the tolerability of the therapy.5
Tornado graphs are one of my favorite advances in the way AEs are presented. It gives a high-level view of how tolerable the therapies are. Lenvatinib plus pembrolizumab is more toxic than sunitinib, but it’s not dramatically different. [What may be] most concerning is a slight increase in the grade 3 or 4 toxicities but having the discontinuation rate at [approximately] 13% should give you comfort in saying these AEs we’re experiencing at a higher level are manageable in the clinic.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 2.2022. Accessed March 1, 2022. https://bit.ly/3vvCSjk
2. Motzer R, Tannir NM, McDermott DF, et al. Conditional survival and 5-year follow-up in CheckMate 214: first-line nivolumab plus ipilimumab (N+I) versus sunitinib (S) in advanced renal cell carcinoma (RCC). Presented at: European Society for Medical Oncology Congress 2021; September 16-21, 2021; virtual. Accessed March 10, 2022. https://bit.ly/3J3zvnx
3. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): results from 42-month follow-up of KEYNOTE-426. J Clin Oncol. 2021;39(suppl 15):4500. doi:10.1200/JCO.2021.39.15_suppl.4500
3. Choueiri TK, Powles T, Burotto M, et al; CheckMate 9ER Investigators. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384(9):829-841. doi:10.1056/NEJMoa2026982
4. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
5. FDA approves nivolumab plus cabozantinib for advanced renal cell carcinoma. FDA. January 22, 2021. Accessed March 1, 2022. https://bit. ly/3tnkoyD
6. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. August 11, 2021. Accessed March 1, 2022 https://bit. ly/3M9j9Mp
7. Choueiri TK, Eto M, Kopyltsov E, et al. Phase III CLEAR trial in advanced renal cell carcinoma (aRCC): outcomes in subgroups and toxicity update. Presented at: European Society for Medical Oncology Congress 2021; September 16-21, 2021; virtual. Accessed March 10, 2022. https://bit. ly/3i19UQc
8. Choueiri TK, Powles T, Porta C, et al. A phase 3 trial of lenvatinib plus pembrolizumab versus sunitinib as a first-line treatment for patients with advanced renal cell carcinoma: overall survival follow-up analysis (the CLEAR study). Presented at: Kidney Cancer Research Summit 2021; October 7-8, 2021; Philadelphia, PA. Accessed March 10, 2022. https://bit.ly/3qNiYNz

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen