Husain Discusses Role of Osimertinib in Non–Small Cell Lung Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Hatim Husain, MD, discussed data on targeted adjuvant therapy for non–small cell lung cancer.
Hatim Husain, MD
Associate Professor
Department of Medicine
University of California San Diego
La Jolla, CA

CASE
- History of present illness: An otherwise healthy White woman (never smoker), aged 60 years, presented with a nonproductive cough.
- Physical examination: ECOG performance status score, 0; blood pressure, 120/93; heart rate, 75; body mass index, 22; lungs, computed tomography angiography bilaterally
- Laboratory results: within normal limits (WNL)
- Chest x-ray: 5.5-cm right mass in right upper lobe (RUL)
- CT chest/abdomen: lobulated 5.5 × 5.1-cm mass in RUL
- Biopsy of RUL: adenocarcinoma, thyroid transcription factor 1 (positive) consistent with non–small cell lung cancer (NSCLC)
- Positron emission tomography: negative for any lymph nodes or distant metastasis
- Brain MRI: negative
- Pulmonary function tests: normal
- Treatment: mediastinoscopy with negative lymph nodes on frozen section, followed by right upper lobectomy without complications
- Pathology: 5.5-cm tumor with negative margins; 0 nodes positive for malignancy (2R, 4R, 7, and 11R are all negative)
- Pathologic stage: confirmed stage IIB* (pT3N0M0) lung adenocarcinoma
- ECOG performance status: 1
- Postoperative therapy course:
- The patient has completed 4 cycles of adjuvant chemotherapy with cisplatin plus pemetrexed.
- Molecular testing: EGFR exon 19 deletion
Targeted Oncology™: What adjuvant therapies are recommended for patients with completely resected NSCLC?
HUSAIN: The rapid recommendation update from the ASCO [American Society of Clinical Oncology] guidelines states that for patients with completely resected stage IB NSCLC, adjuvant osimertinib [Tagrisso] is recommended for patients with an EGFR mutation that has an exon 19 deletion or L858R.
Adjuvant cisplatin [Platinol]-based chemotherapy or atezolizumab [Tecentriq] are not indicated for use in this patient group. For patients with stage IIA, IIB, or IIIA disease, adjuvant cisplatin-based chemotherapy is recommended.
Adjuvant osimertinib is recommended after chemotherapy for those patients with EGFR-sensitizing mutations irrespective of PD-L1 status. A key point is that sometimes [patients with EGFR mutations] can be PD-L1 positive because the oncogene can upregulate, but it is not an immunogenic PD-L1 in many cases. The TKI [tyrosine kinase inhibitor] is indicated irrespective of PD-L1 status. Adjuvant atezolizumab is recommended for all patients with PD-L1 greater than 1% after platinum for those who do not have sensitizing EGFR mutations.1
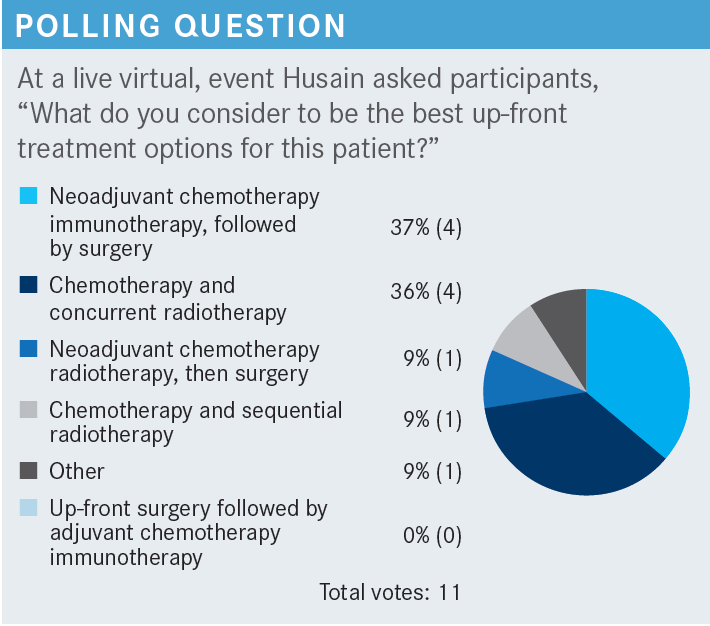
What do we know about adjuvant EGFR-targeted therapy?
Some trials looked at earlier generations of molecules in this space. The RADIANT [NCT00373425] and SELECT [NCT00462995] studies looked at adjuvant erlotinib [Tarceva].2,3 RADIANT was a phase 3 study where erlotinib appeared to have a disease-free survival [DFS] benefit, but it was a retrospective analysis. In the overall intent-to-treat analysis, there was no advantage to erlotinib, and the primary end point was DFS.2 SELECT was a phase 2 study, and all patients received adjuvant platinum-based chemotherapy and then received 2 years of erlotinib.3
It met its primary end point, and at 5 years, 60% of patients were alive and recurrence free. ADJUVANT [NCT01405079] was a Chinese study of patients with stage II and IIIA disease.3 Notably, the primary population had an end point that was looking at risk of recurrence. It is also important to point out that the chemotherapy in this was either vinorelbine [Navelbine] or cisplatin.
The DFS and overall survival [OS] tails of the curve appeared to come together. These [data] lay the foundation for an unmet need, which is further analyses and appropriate end points in the space with third-generation molecules like osimertinib.
What data support the use of osimertinib in patients with completely resected NSCLC?
The ADAURA study [NCT02511106] included patients with completely resected stage IB, stage II, and stage IIIA NSCLC with or without adjuvant chemotherapy.4 Patients had to have preoperative brain imaging, importantly, there was stratification by stage and EGFR mutation status, as well as race. It was a 1:1 randomization. Treatment was continued until disease recurrence or the treatment was completed. The duration of treatment was 3 years and following an independent data monitoring committee recommendation, the study was unblinded due to efficacy.
Even though the results were unblinded, the patients remained blinded at the study sites. Does that change readout of the data?
Yes, that is true. For the demographics, patients were equally distributed across stage IB, stage II, and stage IIIA ranging from 32% to 35%. Also importantly, 60% of patients got adjuvant chemotherapy but 40% did not.5 From the data at the interim analysis, I think some key points are that the changes on the curve show a substantial reduction in the risk of disease recurrence or death in the cohort that receives osimertinib. In fact, it translated to an 83% reduction in the risk of disease recurrence or death and that is in the patients with stage II to IIIA disease. In the overall population, stages IB to IIIA, there was an 80% reduction in the risk of disease recurrence or death.
The updated DFS data are based on the cutoff of April 11, 2022. The median follow-up of osimertinib was 44.2 months, placebo 27.7 months. The hazard ratio is 0.23 [95% CI, 0.18-0.30] and that is in the stage II to IIIA disease category. In the overall population, including stage IB, the hazard ratio is 0.27 [95% CI, 0.21-0.34].6
There was benefit across all the subgroups. I think some key ones just to note are age, race, stage, and EGFR mutation status, whether it be exon 19 deletion or L858R. Another key point is that in both patients who received adjuvant chemotherapy and those who did not, there was an improvement in reducing the risk of disease recurrence or death with osimertinib.7
The updated DFS by stage according to the Seventh Edition AJCC [American Joint Committee on Cancer Staging Manual] shows that the hazard ratio for stage IB was 0.41 [95% CI, 0.23-0.69]; for stage II it was 0.34 [95% CI, 0.23-0.52]; and for stage IIIA it was 0.2 [95% CI, 0.14-0.29]. The Eighth Edition AJCC, the most recent edition, shows that for stage IB, hazard ratio was 0.44 [95% CI, 0.25-0.76]; 0.33 [95% CI, 0.21-0.50] for stage II; and 0.22 [95% CI, 0.15-0.31] for stage IIIA.8
A major site of recurrence in early-stage lung cancer is the brain. An important analysis looked at rates of central nervous system [CNS] response in patients in ADAURA based on the updated CNS data in patients with stage II and IIA disease. The hazard ratio for CNS DFS was 0.24. There was an important separation of the curves at each of the landmark analyses at 24 months, 36 months, and 48 months. The OS data are preliminary, and we are waiting on further long-term data.
What AEs should clinicians be aware of?
The safety data are like what we see in the metastatic setting. Considering the toxicities [on osimertinib] is important, and this includes a discontinuation rate of 11%, dose reduction rate of 9%, and a dose interruption rate of 24%. [There were adverse events (AEs) of grades 3 or higher reported in 20% of patients on osimertinib] and serious AEs were observed in 16% of the study group, but no fatal AEs were reported in this group.
The most common AE that was seen across all grades [for patients on osimertinib] was diarrhea [46%]. The next most common [AEs of any grade on osimertinib] were paronychia [25%], dry skin [23%], pruritus [19%], cough [18%], and stomatitis [18%]. Interstitial lung disease was reported in 11 patients [3%] in the osimertinib group, but all cases were grades 1 or 2. QTc prolongation was reported in 30 patients [9%] in the osimertinib arm and 8 patients [2%] in the placebo arm.
REFERENCES
1. Pisters K, Kris MG, Gaspar LE, Ismaila N; Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA NSCLC Guideline Expert Panel. Adjuvant systemic therapy and adjuvant radiation therapy for stage I-IIIA completely resected non-small-cell lung cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40(10):1127-1129. doi:10.1200/JCO.22.00051
2. Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol. 2015;33(34):4007-4014. doi:10.1200/ JCO.2015.61.8918
3. Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol. 2019;37(2):97-104. doi:10.1200/JCO.18.00131
4. Zhong WZ, Wang Q, Mao WM, et al; ADJUVANT investigators. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol. 2018;19(1):139-148. doi:10.1016/S1470-2045(17)30729-5
5. Spigel DR. Discussion of LBA5. Presented at: 2020 ASCO Virtual Scientific Program; June 1-4, 2020; Virtual. Accessed February 13, 2023. https://bit. ly/3xlsHh3
6. Herbst RS, Tsuboi M, John T, et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB-IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. J Clin Oncol. 2020;38(suppl 18):LBA5. doi:10.1200/ JCO.2020.38.18_suppl.LBA5
7. Wu YL, Tsuboi M, He J, et al; ADAURA Investigators. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
8. Tsuboi M, Wu Y, Grohe C, et al. Osimertinib as adjuvant therapy in patients (pts) with resected EGFR-mutated (EGFRm) stage IB-IIIA non-small cell lung cancer (NSCLC): updated results from ADAURA. Ann Oncol. 2022;33(suppl 7):S808-S869. doi:10.1016/annonc/annonc1089

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More