HRD Biomarker Predicts Response to Neoadjuvant Chemotherapy in TNBC
Neoadjuvant chemotherapy with carboplatin has been shown to improve the pathologic complete response rate for patients with triple negative breast cancer.
Homologous recombination deficiency (HRD) served as a predictive biomarker for identifying patients with triple-negative breast cancer (TNBC) who would benefit from neoadjuvant carboplatin-based chemotherapy, according to an analysis of results from the phase 2 NeoCART trial (NCT03154749).1
The study authors, led by Liulu Zhang, MD, of the Cancer Center at Guangdong Provincial People’s Hospital and Guangdong Academy of Medical Sciences in Guangzhou, China, noted that HRD could also help with screening patients for the appropriate chemotherapy backbone to be combined with immunotherapy.
“HRD is a potential predictive biomarker for patient clinical benefit from neoadjuvant platinum-based chemotherapy and provides a possibility for screening the required chemotherapy backbone for combination with immunotherapy and patients who are most likely to benefit from neoadjuvant immunochemotherapy,” Zhang et al wrote in their report, published in JCO Precision Oncology.
Putting the Scene Into Context
Neoadjuvant chemotherapy with carboplatin has been shown to improve the pathologic complete response (pCR) rate for patients with TNBC who have a tumor larger than 1 cm or axillary lymph node involvement. “There is no doubt that platinum plays an important role in TNBC [neoadjuvant chemotherapy],” Zhang et al wrote.
However, carboplatin can also bring in added hematologic toxicity, making it important to identify which patients will benefit from its addition. Patients with HRD tumors may be more sensitive to carboplatin because it triggers damaged DNA crosslinks.
The multicenter, randomized, controlled, open-label NeoCART trial explored the use of 6 cycles of docetaxel and carboplatin compared with 4 cycles of epirubicin and cyclophosphamide followed by 4 cycles of docetaxel for patients with stage II or III TNBC treated in the neoadjuvant setting. In the primary analysis, the pCR rate, the primary end point, was 61.4% with the carboplatin regimen (n=44) compared with 38.6% with the epirubicin regimen (n=44; OR, 2.52; 95% CI, 2.4-43.1; P for noninferiority=.004). Noninferiority was achieved for carboplatin plus docetaxel compared with epirubicin and cyclophosphamide.2
In a previous meta-analysis of 7 clinical trials led by Zhang, neoadjuvant chemotherapy with platinum led to a pCR benefit in patients with HRD compared with patients without HRD (OR, 3.84; 95% CI, 1.93-7.64; P =.0001). On the other hand, those who did not receive platinum did not show a difference in pCR rates by HRD.3
Additionally, pCR rates did not differ significantly by BRCA mutation status in those patients with HRD (OR, 2.00; 95% CI, 0.77-5.23; P =.16). However, patients without HRD with wild-type BRCA did not perform as well on carboplatin as patients with HRD (OR, 3.64; 95% CI, 1.83-7.21; P =.0002).3
For patients with high-risk TNBC, neoadjuvant immunochemotherapy has become the standard of care, capable of providing greater responses for this patient population than neoadjuvant chemotherapy alone. Results from past trials of neoadjuvant immunochemotherapy have also shown that the use of carboplatin contributes to higher pCR rates. For example, the KEYNOTE-173 trial (NCT02622074) looked at the use of pembrolizumab, a PD-1 inhibitor, in combination with a taxane with or without carboplatin followed by doxorubicin and cyclophosphamide leading up to surgery in patients with high-risk, early-stage, nonmetastatic TNBC. In groups of patients who received carboplatin, the rate of pCR was slightly higher for those who received platinum every 3 weeks compared with those who received it weekly (63% vs 55%).4 Tumors with HRD are considered more sensitive to immunotherapy because of higher levels of somatic mutations and higher tumor mutational burden.1
HRD Analysis
Investigators conducted a preplanned analysis of HRD and other potential biomarkers as predictors of benefit for the investigational combination regimen. About half of the patients were analyzed for HRD (n=43), with 13 patients (59%) having high HRD scores in the carboplatin arm and 17 patients (80%) having high HRD scores in the epirubicin arm. Tumor BRCA mutations were reported in 4 patients (18.2%) in the investigational arm and 3 patients (14.3%) in the control arm. Other commonly mutated genes included TP53 (93%); PIK3CA (27.9%); PTEN (11.6%); and ATRX, NF1, PIK3R1,and PKHD1 (9.3% each).
For patients with HRD, the median age was 50 years, and 44.3% had lymph node involvement. Median tumor mutational burden was 5.3 mut/Mb (range, 0-14.8), and the median percentage of tumor-infiltrating lymphocytes (TILs) was 10%, although approximately 23% of patients had stromal TIL levels of more than 30%.
A total of 59.1% of evaluable HRD patients in the carboplatin arm achieved pCR vs 33.3% in the epirubicin arm. The rate of residual cancer burden of 0 or 1 was 63.6% with the carboplatin regimen and 38.1% with the epirubicin regimen. In the carboplatin arm, patients who achieved pCR tended to have higher HRD scores than those who did not achieve pCR (P =.061). A similar effect was seen with residual cancer burden rates. In the epirubicin arm, however, no associations were seen between HRD scores and pCR or residual cancer burden.
Half of all patients with BRCA1/2 tumor or germline mutations achieved a pCR in each arm (FIGURE). Nonetheless, an association was not found between BRCA1/2 mutation status and pCR.
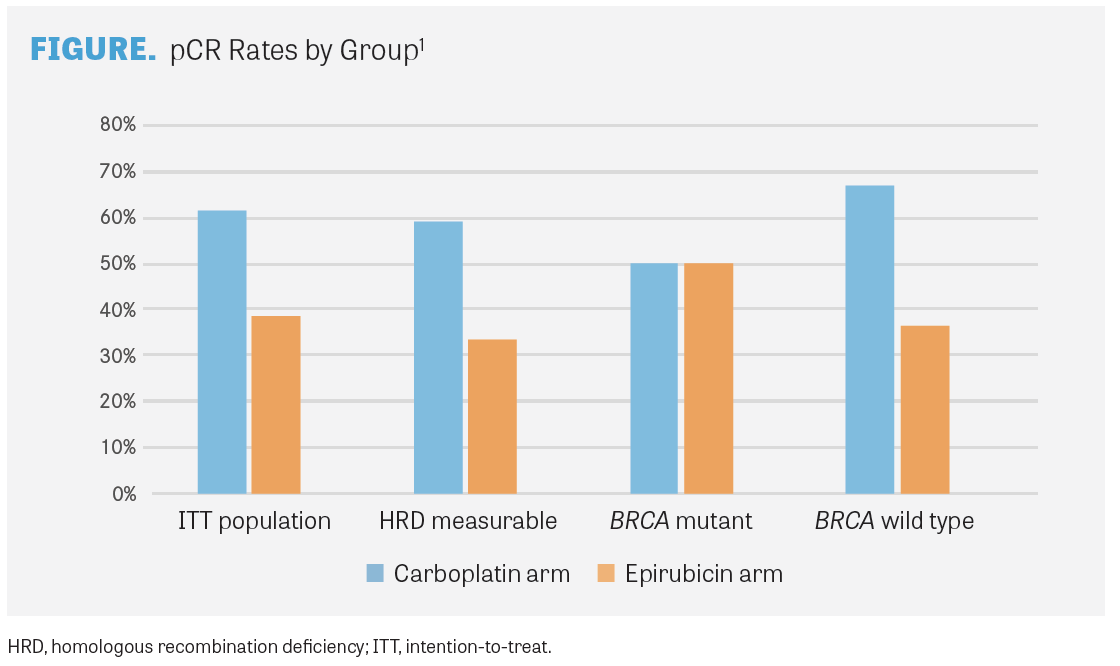
Responses in the carboplatin arm were also associated with TILs (OR, 1.101; P =.042). By multivariate analysis, HRD score was not associated with pCR for the carboplatin arm or the total patient population. Tumor mutation burden did not show a correlation with response to neoadjuvant chemotherapy in either treatment arm.
The study authors noted that the analysis was a preplanned retrospective analysis of the study, and because HRD was not a primary end point of the study, the results should be considered with some caution. Additionally, overall survival data for the study are not yet mature, so the effect of HRD on long-term survival cannot yet be seen.
REFERENCES:
1. Zhang L, Wu Z, Li J, et al. Impact of homologous recombination deficiency on outcomes in patients with triple-negative breast cancer treated with carboplatin-based neoadjuvant chemotherapy: secondary analysis of the NeoCART randomized clinical trial. JCO Precis Oncol. 2023;7:e2200337. doi:10.1200/PO.22.00337
2. Zhang L, Wu ZY, Li J, et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label phase II trial. Int J Cancer. 2022;150(4):654-662. doi:10.1002/ijc.33830
3. Zhang L, Chen Y, Cheng MY, et al. Homologous recombination deficiency predicts the response to platinum-based neoadjuvant chemotherapy in early-stage triple-negative breast cancer patients: a systematic review and meta-analysis. Ther Adv Med Oncol. 2022;14:17588359221096253. doi:10.1177/17588359221096253
4. Schmid P, Salgado R, Park YH, et al. Pembrolizumab plus chemotherapy as neoadjuvant treatment of high-risk, early-stage triple-negative breast cancer: results from the phase 1b open-label, multicohort KEYNOTE-173 study. Ann Oncol. 2020;31(5):569-581. doi:10.1016/j.annonc.2020.01.072
