Grothey Emphasizes Multidrug Therapies in mCRC
The use of more aggressive frontline chemotherapy regimens plus biologics for the treatment of patients with meta-static colorectal cancer may be warranted based on mutational status, tumor sidedness, treatment goals, prognosis, and patient disposition, according to a presentation by Axel Grothey, MD, at the 14th Annual New Orleans Summer Cancer Meeting, held July 19 to 21, 2019, in Louisiana.
Axel Grothey, MD

Axel Grothey, MD
The use of more aggressive frontline chemotherapy regimens plus biologics for the treatment of patients with meta-static colorectal cancer (mCRC) may be warranted based on mutational status, tumor sidedness, treatment goals, prognosis, and patient disposition, according to a presentation by Axel Grothey, MD, at the 14th Annual New Orleans Summer Cancer Meeting, held July 19 to 21, 2019, in Louisiana.
“It’s not a one-size-fits-all approach, and we need to start individualizing therapy,” Grothey said. “There are a lot of factors we can take into account, including patient characteristics [such as age], comorbidities, and goal of therapy. Then, of course, there are molecular characteristics like standardRASandBRAFmutations, MSI [microsatellite instability] status, and potentiallyHER2; all [of] this is being used to integrate our treatment approaches.”
Grothey, director of gastrointestinal cancer research at West Cancer Center Research Institute in Germantown, Tennessee, highlighted the newest data for treatments of mCRC and recent trials involving frontline triplet chemotherapy and immunotherapeutics.1
Candidates for FOLFOXIRI Plus Bevacizumab
Upfront treatment with folinic acid/fluorouracil/oxaliplatin/ irinotecan (FOLFOXIRI) plus bevacizumab (Avastin) has shown favorable efficacy compared with doublet therapy in clinical trials, but concerns exist regarding its toxicity and the feasibility of subsequent treatments following progression.
TRIBE2 was an open-label, multicenter, randomized phase III trial in patients with unresectable and previously untreated mCRC that evaluated folinic acid/fluorouracil/ oxaliplatin (FOLFOX) plus bevacizumab in the frontline followed by folinic acid/fluorouracil/irinotecan plus bevacizumab in the second line versus FOLFOXIRI plus bevacizumab as both first- and second-line therapies. The primary endpoint was progression-free survival 2 (PFS2), defined as the time from randomization until second evidence of disease progression on any treatment.2 The trial met its primary endpoint, with a median PFS2 of 18.9 months in the FOLFOXIRI arm versus 16.2 months in the control arm (HR, 0.69; 95% CI, 0.57-0.83;P<.001).1
Regarding sidedness, the triplet improved outcomes predominantly in patients with right-sided tumors. The hazard ratios for overall survival and PFS for the triplet and doublet therapies were 0.56 and 0.59, respectively; corresponding hazard ratios for left-sided tumors were 0.99 and 0.89, showing no statistically significant improvements with triplet therapy compared with doublet therapy added to bevacizumab.
“Right-sided cancers are more aggressive. Prior analysis of the initial TRIBE study [NCT00719797], which actually investigated the triplet therapy compared with doublet, showed the survival benefit for triplet was clearly seen in right-sided cancers,” Grothey said.
Another phase III trial, VISNU-1, looked at patients ≤70 years with ≥3 circulating tumor cells (CTCs) at baseline andKRASwild-type disease. Patients received either FOLFOX or FOLFOXIRI plus bevacizumab in the front line.
The study met its primary endpoint of median PFS superiority in the FOLFOXIRI group versus the FOLFOX group (12.4 vs 9.3 months; HR, 0.64; 95% CI, 0.49-0.82;P= .0006), suggesting the triplet regimen plus bevacizumab could be a treatment option for patients with mCRC and ≥3 CTCs.3
FOLFOXIRI With Panitumumab in Both Left-Sided and Right-Sided Cancers
The antiepidermal growth factor receptor fully human monoclonal antibody panitumumab (Vectibix) added to FOLFOXIRI may be active in patients with both left-sided and right-sided as well asBRAF-mutant mCRC.
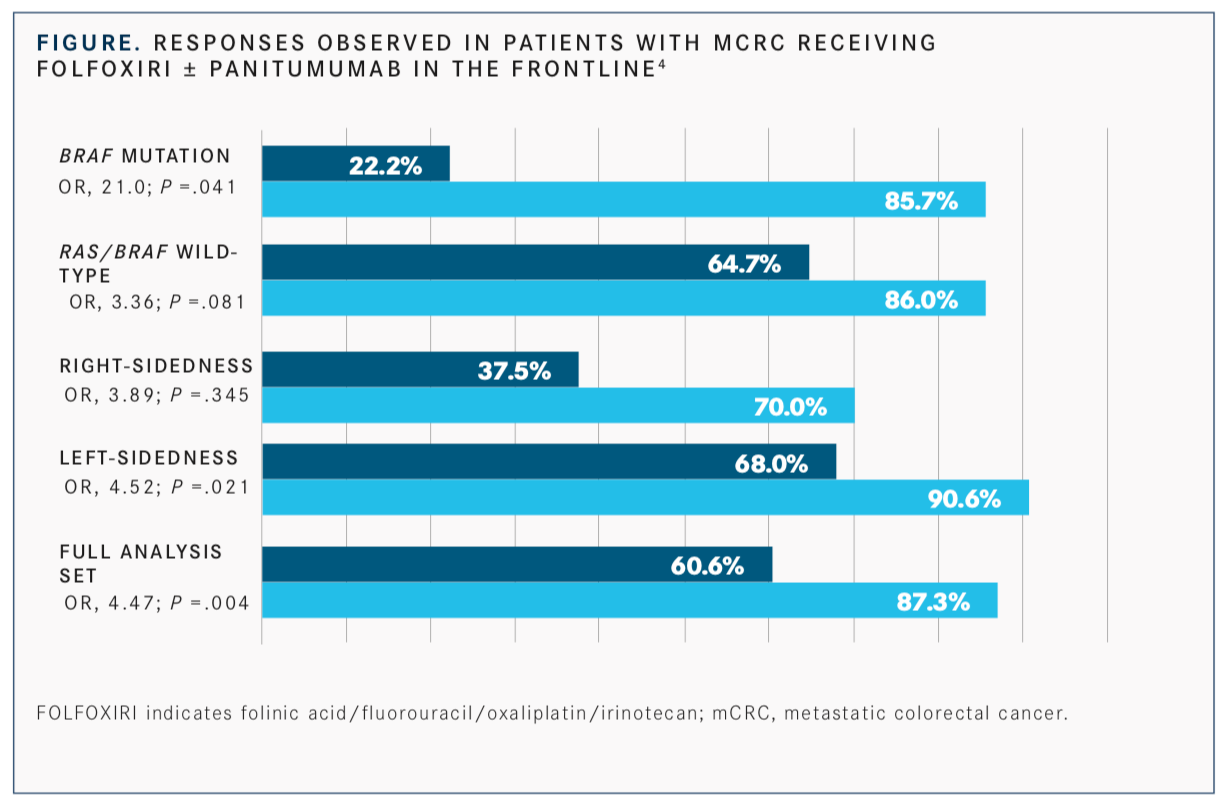
The VOLFI phase II trial in unresectableRASwild-type mCRC randomized 96 patients 2:1 to either FOLFOXIRI alone or with panitumumab administered every 2 weeks. Treatment lasted until progressive disease, resectability, or a maximum of 12 cycles.4
The primary endpoint of objective response rate was 87.3% with FOLFOXIRI plus panitumumab versus 60.6% with FOLFOXIRI alone. In 78 patients with left-sided tumors only, the ORR was 90.6% with FOLFOXIRI plus panitumumab and 68% with FOLFOXIRI. A total of 18 patients had right-sided tumors, and of those, 70% achieved an objective response with the panitumumab regimen versus 37.5% with FOLFOXIRI alone (FIGURE).4
In patients withRAS/BRAFwild-type disease, the objective response rate (ORR) was 86% with FOLFOXIRI plus panitumumab and 64.7% with FOLFOXIRI. Patients on the panitumumab arm with aBRAFmutation had an 85.7% ORR versus 22.2% with FOLFOXIRI.4
Grothey noted that the impressive 91% response rate in patients with left-sided tumors is high and not frequently seen in this patient population.
“Triplets are underappreciated and underutilized. There are a lot more indications for triplets than one might think,” Grothey concluded.
References
- Grothey, A. Colon cancer: are we making progress? Presented at: 14th Annual New Orleans Summer Cancer Meeting; July 19-21, 2019; New Orleans, LA.
- Cremolini C, Antoniotti C, Lonardi S, et al. Updated results of TRIBE2, a phase III, randomized strategy study by GONO in the first- and second-line treatment of unresectable mCRC.J Clin Oncol. 2019;37(suppl 15; abstr 3508). doi: 10.1200/JCO.2019.37.15_suppl.3508.
- Sastre J, Vietiez JM, Gomez-Espaῆa MA, et al. Randomized phase III study comparing FOLFOX + bevacizumab versus folfoxiri + bevacizumab (BEV) as 1st line treatment in patients with metastatic colorectal cancer (mCRC) with ≥3 baseline circulating tumor cells (bCTCs).J Clin Oncol. 2019;37(suppl 15; abstr 3507). doi: 10.1200/JCO.2019.37.15_suppl.3507.
- Geissler M, Riera-Knorrenschild J, Martens UM, et al. Final results and OS of the randomized phase II VOLFI trial (AIO-KRK0109): mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer (mCRC).J Clin Oncol.2019;37(suppl 15; abstr 3511). doi: 10.1200/JCO.2019.37.15_suppl.3511.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More