GI Study Addresses Tumor Molecular Heterogeneity Using Unique Trial Designs
Research characterizing the relationship between this heterogeneity and response to targeted therapies can lead to improved selection of patients who will benefit from specific therapies.
Daniel Catenacci, MD

Addressing tumor molecular heterogeneity is a challenge, especially when designing effective biomarker-driven treatment strategies. Research characterizing the relationship between this heterogeneity and response to targeted therapies can lead to improved selection of patients who will benefit from specific therapies. This, in turn, may improve response rates to targeted therapies, some of which have been disappointingly low in unselected patient populations.
“We recognize that many differences exist [in genomic alterations between different patients with gastroesophageal adenocarcinoma], something we call interpatient molecular heterogeneity, and that has led to some hurdles in trying to study given genetic events and matched targeted therapy, especially when it involves a smaller subset of the whole … and [it] has been prohibitive and difficult to accomplish in many situations or scenarios,” said Daniel Catenacci, MD, during an interview with Targeted Therapies in Oncology. He is associate professor of medicine and director of the GI oncology program at the University of Chicago in Illinois.
Basing therapy selection in metastatic gastroesophageal adenocarcinoma (GEA) on analysis of the primary tumor alone may be suboptimal because of intrapatient heterogeneity of key clinical biomarkers; according to published reports, genomic profiling discrepancies between the primary tumor and lymph node metastases are observed in approximately 30% to 50% of cases.1,2 The extent of genomic variability in patients with newly diagnosed metastatic GEA was evaluated in a study using sequencing to compare genomic alterations between the primary tumor and paired metastatic lesions.2 In cohort 1 (n=11 patients), a comparison between primary tumors and metastases at baseline revealed discrepant pathogenic alterations between the 2 sites in 45% of patients. Evaluation of samples from multiple regions of the primary tumor and metastatic sites in cohort 2 (n=26 patients) demonstrated striking heterogeneity both within the primary tumor and between primary and metastatic sites. In cohort 3 (n=11 patients), circulating tumor DNA (ctDNA) from blood plasma was compared with standard primary tumor samples. The results revealed genomic alterations present in the primary tumor but not in the ctDNA, and vice versa. Overall, given the wide extent of tumor heterogeneity, this study’s findings highlighted the importance of performing biomarker profiling on samples from several regions of multiple tumor sites to optimize targeted therapy selection in trials for metastatic GEA.2
“One strategy to address [the limitation of infrequent driver alterations] has been to use basket studies or a tissue-agnostic approach that pooled [results] across different tumor types. As long as tumors had a given genetic mutation, you would be able to enroll patients to that cohort,” said Catenacci.
Expansion platform designs have been evaluated for clinical trials of targeted therapies in oncology, with the majority employing type I expansion platform designs. These “umbrella trials” assign patients to cohorts that pair a specific tumor molecular profile with a matched targeted treatment. Because each cohort has an individual end point, a large sample is typically required for sufficient statistical power.1 “The limitations of type I expansion platform designs [include] that generally, they have been testing 1 drug, a monotherapy. It has been hard to combine therapies, especially standard therapy or chemotherapy, which differs across tumor types. It is hard to design such studies, so by default [the patient population in the study is heavily pretreated and receiving monotherapy, which is suboptimal]. In addition to that, there are still usually small cohorts and nonrandomized trials,” Catenacci said.
Alternatively, type II expansion platform designs pool multiple biomarker-drug pairings, allowing for smaller sample sizes. Successful type II trial design depends on use of a correct predefined treatment algorithm, appropriate biomarker-drug pairings, and validated companion diagnostics. Type II expansion platform designs can either be histology dependent (type IIA) or histology agnostic (type IIB).1
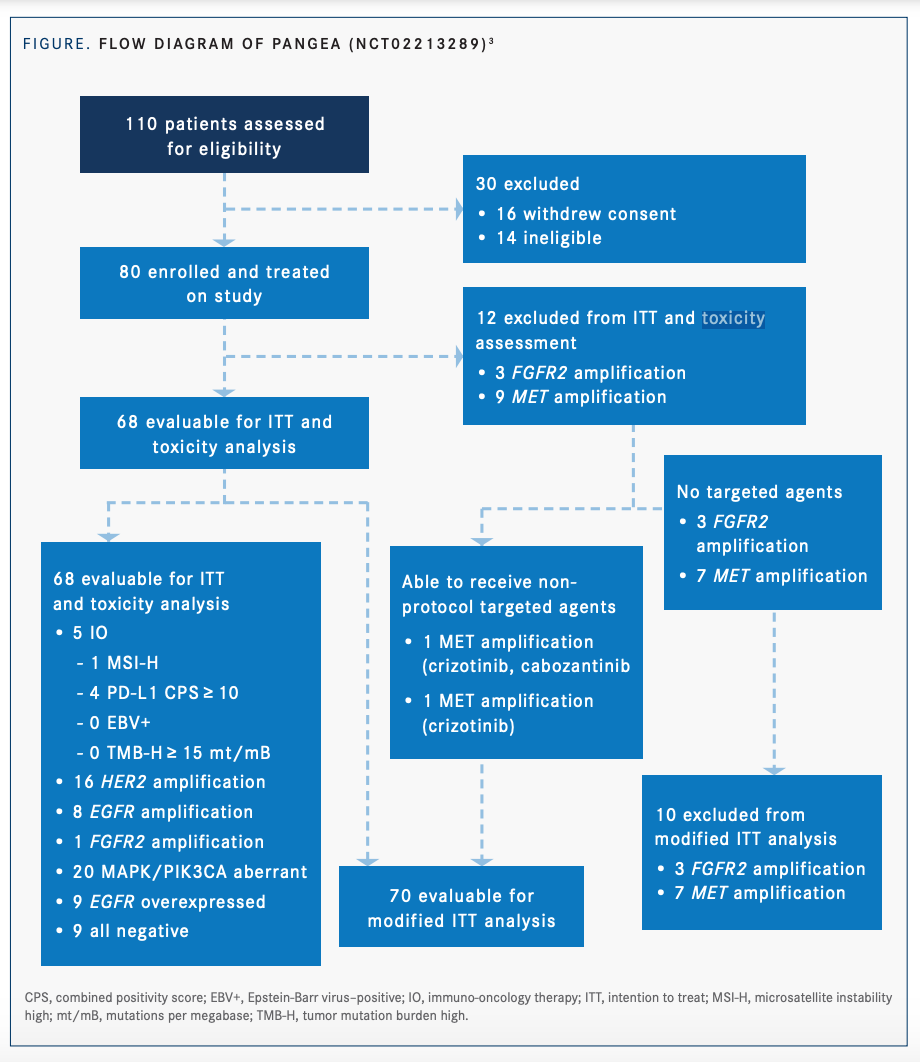
An example of a type IIA expansion platform design is the PANGEA study (NCT02213289).3,4 This phase 2, open-label, single-arm trial aimed to address molecular heterogeneity at baseline and upon disease progression using a personalized treatment strategy (FIGURE3,4). Patients with newly diagnosed advanced GEA were included in the study, regardless of biomarker status. Biomarker profiling was performed on primary tumor and metastatic biopsies at baseline, upon first disease progression, and upon second disease progression. Testing of ctDNA, a noninvasive means of biomarker analysis, was performed at baseline and at each point in disease progression. Eight treatment groups were paired with 6 therapeutic targeted monoclonal antibodies.3,4
“An important point to note is that in the biomarker groups, there’s overlap in a given patient, so a single patient’s tumor could fall into more than 1 group in our study. We prioritized the biologic groups such that if a patient’s tumor did fall into more than 1 group, then we had already prespecified what would happen in this treatment algorithm, [making] this more reproducible by others,” Catenacci said.
“Our strategy was that all patients screened would [receive a targeted antibody], and it would be prioritized based on their biology and tested prospectively,” Catenacci added.
All patients in the study received chemotherapy in combination with targeted monoclonal antibody therapy based on biomarker analysis. In the event of discordant biomarker profiles between the metastatic site and the primary tumor, the former took precedence in treatment assignment. Reassignment to a new line of sequential therapy based on biomarker analysis could occur at each point in disease progression.3,4
Of 80 enrolled patients, 68 were included in the intention-to-treat (ITT) analysis. The primary efficacy end point was met, with 1-year overall survival (OS) of 69.4% (P <.001).4 The median OS was 16.4 months (95% CI, 13.8-20.8).4 Median OS for the ITT patients was significantly longer in the higher-priority biological groups (1-4) compared with the lower-priority groups (6-8), at 25.8 months vs 13.9 months, respectively (P =.002).3 At baseline, spatial heterogeneity was observed in 37% of patients, who had discordant molecular profiles between primary and metastatic tumors.4 Of the 55 patients in the modified ITT group who progressed on first-line therapy, 27 (49%) were assigned to a different biomarker group for second-line therapy due to temporal biomarker evolution. Similarly, 13 of 27 patients (48%) with second-line progression changed group assignment for third-line treatment. This novel treatment strategy, with sequential antibody assignments matched to biomarker profiles, demonstrated improved efficacy outcomes in GEA compared with historical controls.3
“A limitation of [type II expansion platform design] is that just because the strategy is considered successful in a positive study doesn’t necessarily mean that each subarm of the study contributed to that outcome… that’s why we looked at the outcome by arm, [which was] prespecified to show that each arm was at least contributing in some way to the outcome,” Catenacci said.
Cathy Eng, MD

Cathy Eng, MD, professor of medicine (hematology and oncology), codirector of GI oncology, and coleader of the Gastrointestinal Cancer Research Program at Vanderbilt-Ingram Cancer Center in Nashville, Tennessee, commented that PANGEA featured “an innovative approach. I think looking at resistance and how to provide sequencing of therapy is the most interesting [concept from the trial] from my perspective as a clinician and clinical researcher.”
An example of a type IIB expansion platform design is SHIVA (NCT01771458), the first study to evaluate off-label use of molecularly targeted agents. This open-label, randomized, multicenter, phase 2 trial included patients with any metastatic solid malignancy refractory to standard-of-care therapy.5 Patients with tumor genetic alterations in hormone receptor, PI3K/AKT/mTOR, or RAF/MEK pathways were randomized to receive either 1 of 11 matched targeted agents (experimental group; n = 99) or physician’s choice of treatment (control group; n = 96). The median progression-free survival (PFS) was 2.3 months in the experimental group and 2.0 months in the control group, (HR, 0.88; 95% CI, 0.65-1.19; P = .41). Although the trial did not demonstrate improved PFS with the use of targeted agents outside their approved indications, it was a pivotal step in the exploration of type II expansion platform study design.5
“A limitation is that the conclusion [of the SHIVA trial] should not be that subarms are negative,” Catenacci said. “The conclusion should be that the overall approach was negative…There may have been 1 or more subgroups within that SHIVA strategy that are in fact truly better, yet they have to be proven in a different way or [with] a different strategy.”
Despite the acknowledged limitations to the type II expansion platform design, it has several benefits, including the ability to evaluate tumor evolution over time and to incorporate sequential treatment after disease progression.1 Another benefit of the type II expansion platform design is that it allows for a small sample size, which is typically a barrier when studying uncommon molecular alterations. A smaller sample size is allowed because results are pooled within a single trial. Because patients receive maximum exposure to the appropriate agents, a smaller sample size is needed to achieve statistical significance.1
Type II expansion platform trials are advantageous for patients because there is a plan from the beginning, Eng said, “so it brings confidence to the patients. Also, the patient is being followed by the same research team, [which I] think is to the patient’s advantage…It’s not like a randomized phase 3 study [in which the patient is] switched from one trial to another trial [after disease progression] and you’re only following the patient for overall survival.”
One example of a trial with a traditional design is RAINFALL (NCT02314117), a randomized, placebo-controlled, double-blind, multicenter, phase 3 trial in patients with metastatic GEA. Patients received fluoropyrimidine and cisplatin plus either ramucirumab (Cyramza; n=326) or placebo (n=319).6 Median PFS was significantly longer in the ramucirumab group (5.7 months) compared with the placebo group (5.4 months; HR, 0.753; 95% CI, 0.607-0.935; P =.011).
RAINFALL, Catenacci said, was “what I would call a traditional phase 3 study. It wasn’t selecting patients based on any biology to predict benefit of a given drug and just assessing to see if it was better.”
Various factors are considered when choosing the appropriate design for a clinical trial, Catenacci said. “It’s dependent on disease type, on details of the setting that is being investigated, and on the drugs that are going to be investigated. The hypothesis associated with some drugs may be that they work similarly across all subgroups of a given disease, and in that case, a traditional study would make a lot of sense. On the other hand, if there are drugs that are only hypothesized to work in certain select situations and biological circumstances within a disease, then that’s where an expansion platform design might be more useful.”
References:
1. Catenacci DVT. Next-generation clinical trials: novel strategies to address the challenge of tumor molecular heterogeneity. Mol Oncol. 2015;9(5):967-996. doi:10.1016/j.molonc.2014.09.011
2. Pectasides E, Stachler MD, Derks S, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018;8(1):37-48. doi:10.1158/2159-8290.CD-17-0395
3. Catenacci DVT, Moya S, Lomnicki S, et al. Personalized Antibodies for Gastroesophageal Adenocarcinoma (PANGEA): a phase II study evaluating an individualized treatment strategy for metastatic disease. Cancer Discov. 2021;11(2):308-325. doi:10.1158/2159-8290.CD-20-1408
4. Catenacci DVT, Lomnicki S, Chase L, et al. Personalized ANtibodies for GastroEsophageal Adenocarcinoma (PANGEA): primary efficacy analysis of the phase II platform trial (NCT02213289). J Clin Oncol. 2020;38(suppl 4):abstr 356. doi:10.1200/JCO.2020.38.4_suppl.356
5. Le Tourneau C, Delord J-P, Gonçalves A, et al; SHIVA Investigators. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324-1334. doi:10.1016/S1470-2045(15)00188-6
6. Fuchs CS, Shitara K, Di Bartolomeo M, et al; RAINFALL Study Group. Ramucirumab with cisplatin and fluoropyrimidine as first-line therapy in patients with metastatic gastric or junctional adenocarcinoma (RAINFALL): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):420-435. doi:10.1016/S1470-2045(18)30791-5
