Further Analyses Expand Upon Duration of Benefit With Sotorasib in KRAS G12C+ NSCLC
Following the FDA approval of sotorasib for the treatment of KRAS G12C-positive non–small cell lung cancer, there is a great deal of interest in the KRAS inhibitor and its potential benefit.
Suresh S. Ramalingam, MD

As sotorasib (Lumakras) is the first agent to be FDA approved for the treatment of patients with KRAS G12C–mutant non–small cell lung cancer (NSCLC), there is a great deal of interest in the KRAS inhibitor and its potential benefit.
The FDA approved sotorasib for patients with locally advanced or metastatic NSCLC harboring a KRAS G12C mutation who have previously received at least 1 systemic therapy. The agency’s decision was based upon results from the phase 1/2 CodeBreaK 100 trial (NCT03600883).1 The study showed an objective response rate (ORR) of 37.1% (95% CI, 28.6%-46.2%), a median progression-free survival (PFS) of 6.8 months (95% CI, 5.1-8.2), and a median overall survival (OS) of 12.5 months (95% CI, 10.0-not estimable) among participants.2
Following the regulatory decision, analyses of CodeBreaK 100 continue to further investigate patient and disease characteristics for factors that could affect potential benefit from this first-in-class KRAS inhibitor.
Two analyses presented during the International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer offered some characteristics that could lead to hypothesis-generating variations in clinical response patterns and duration of benefit from treatment with sotorasib in patients with KRAS G12C–mutant NSCLC.3,4
Brain Metastases
Sotorasib elicited intracranial complete responses (CRs) and continued intracranial stabilization in patients with KRAS G12C–mutated NSCLC and stable brain metastases who had previously been treated with radiation or had undergone surgery, according to a posthoc analysis of the CodeBreaK 100 trial.3
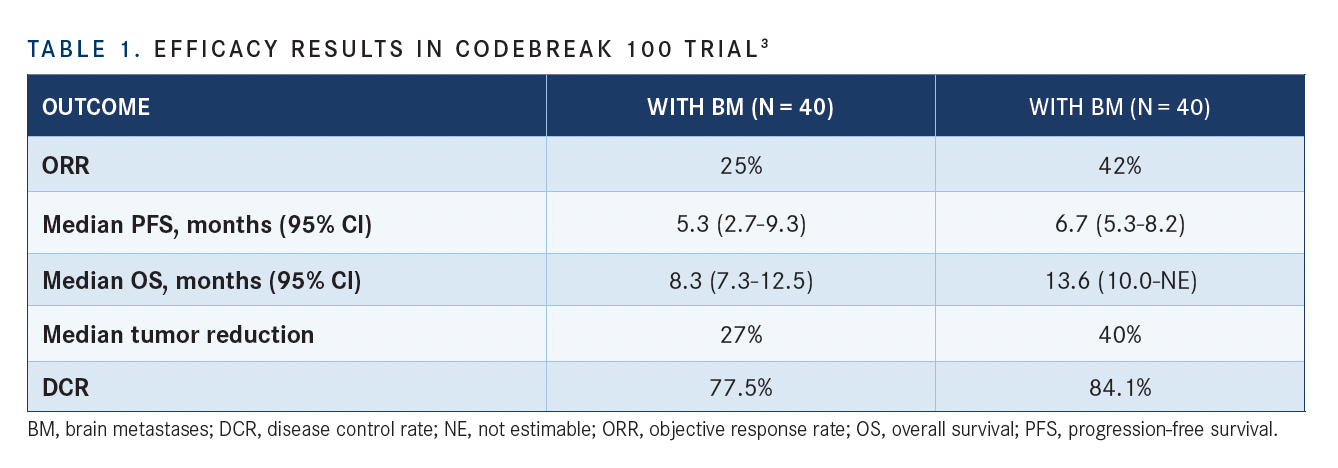
At a median follow-up of 12 months, sotorasib led to a confirmed ORR per RECIST 1.1 criteria of 25% in patients with NSCLC and baseline brain metastases (n = 40) compared with 42% in patients without baseline brain metastases (n = 132). The disease control rates (DCRs) were 77.5% vs 84.1%, respectively (TABLE).
Additionally, the median PFS was 5.3 months (95% CI, 2.7-9.3) in patients with brain metastases compared with 6.7 months (95% CI, 5.3-8.2) in those without brain metastases. The median OS was 8.3 months (95% CI, 7.3-12.5) vs 13.6 months (95% CI, 10.0-not estimable), respectively.
Notably, the intracranial DCR was 88% among 16 patients with evaluable brain metastases.
“Brain metastases are common in patients with NSCLC, [occurring in 30% to 50% of patients], and are associated with poor outcomes,” wrote Suresh S. Ramalingam, MD, and colleagues in a presentation of the data.4 The standard of care for patients with NSCLC and brain metastases is docetaxel plus ramucirumab (Cyramza), which is associated with median PFS estimates of 3 to 5 months.5
“[These data] provide the first evaluation of sotorasib in patients with NSCLC and stable brain metastases,” added Ramalingam, a professor of hematology and medical oncology and Roberto C. Goizueta Chair for Cancer Research at Emory University School of Medicine, and executive director of Winship Cancer Institute of Emory University in Atlanta, Georgia.
Patients with active brain metastases were excluded from the CodeBreaK 100 trial; however, patients with stable, asymptomatic brain metastases were eligible for inclusion.
In the posthoc analysis, the investigators retrospectively evaluated responses to sotorasib in target and nontarget stable brain metastases. They defined target lesions as measurable lesions suitable for accurate repeated measurements, and nontarget lesions as lesions too small for accurate repeated measurements.
In patients with baseline brain metastases (n = 40), the median age was 67 years (range, 46-83), and 98% of patients were current or former smokers. Most patients had an ECOG performance status of 1 (75%), and nearly a quarter had 3 metastatic sites (27%). Sixteen patients (40%) had received 1 prior line of systemic anticancer therapy. Regarding local therapies for the brain, 65% of patients had received prior radiotherapy, 20% had undergone surgery, and 12% had received radiotherapy and surgery.
In patients without baseline brain metastases (n = 134), the median age was 65 years (range, 37-86), and 90% of patients were current or former smokers. Most patients had an ECOG performance status of 1 (71%) and 1 metastatic site (45%) and had received 1 prior line of systemic anticancer therapy (41%). Regarding local therapies for the brain, 2 patients had received prior radiotherapy and 1 had undergone surgery. Of note, these local therapies may have been historic and have led to sufficient removal of the brain metastasis so that the patient did not have a confirmed brain metastasis at baseline during the time of enrollment.
Additional findings from the study demonstrated that the median tumor reduction rate was 27% in patients with brain metastases compared with 40% in patients without brain metastases.
Central Response Assessment in Neuro-Oncology (RANO) analysis demonstrated that 9.2% (n = 16/174) of patients had baseline scans, as well as at least 1 on-treatment evaluable scan; this confirmed that 9 patients had 1 lesion, 2 had 4 lesions, and 5 had 5 or more lesions.
In patients with target and nontarget central nervous system (CNS) lesions (n = 3), none achieved a CR, 33% (n = 1) achieved stable disease (SD), and 67% (n = 2) had progressive disease (PD). In patients with nontarget CNS lesions (n = 13), 15% (n = 2) achieved a CR, 85% (n = 11) achieved SD, and none had PD. In all evaluable patients with brain metastases per RANO analysis (n = 16), 13% (n = 2) achieved a CR, 75% (n = 12) achieved SD, and 13% (n = 2) had PD.
Regarding safety, 20% (n = 8) of patients with baseline brain metastases reported grade 3 treatment-related adverse effects (TRAEs) with sotorasib compared with 19% (n = 26) of patients without baseline brain metastases. Grade 4 TRAEs were reported in 0 patients vs 2 patients (1.5%), respectively. No grade 5 TRAEs occurred in either arm.
The ongoing phase 1/2 CodeBreaK 101 trial (NCT04185883) is evaluating sotorasib in combination with other anticancer therapies including pembrolizumab (Keytruda), trametinib, and more, in patients with KRAS G12C– mutated advanced solid tumors and active untreated brain metastases.
Presence of Co-Mutations
One such exploratory analysis from the trial suggested that the clinical response to sotorasib could vary in patients with KRAS G12C–mutated NSCLC, depending on their genomic profiles and co-mutational status.4
“Recently, diverse and frequently coexisting mechanisms of acquired resistance to KRAS inhibitors were reported, including secondary alterations in KRAS itself, as well as other components of the RT kinase/ MAP kinase pathways,” said Ferdinandos Skoulidis, MD, PhD.
Investigators conducted tissue analysis and looked at the following genomic profiles in 65 of 126 enrolled patients who had baseline tissue samples and at least 3 months’ follow-up: KEAP1 (n = 11); STRK11 (n = 13); RTK genes (n = 39); cell cycle genes (n = 27); DNA damage response (DDR) genes (n = 50); PI3K/AKT/mTOR (n = 24), , and WNT (n = 24) pathway genes; and RAS/MAPK pathway genes beyond KRAS G12C (n = 23).
A total of 33.8% of patients (n = 22) were early progressors, meaning that their disease progressed before 3 months; 35.4% (n = 23) were late progressors (event of progressive disease at or after 3 months); and 30.8% (n = 20) had ongoing disease control. However, the results varied by co-mutational subgroups (FIGURE).
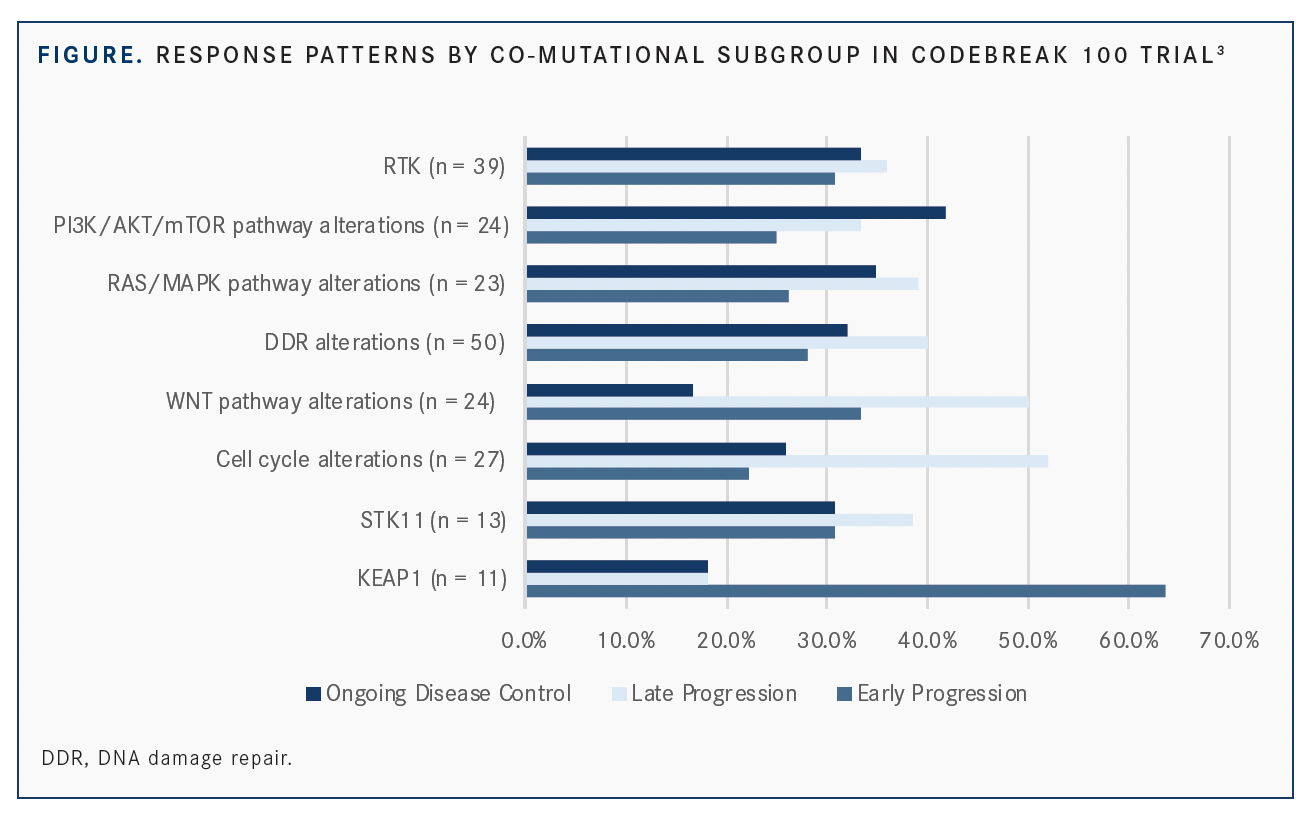
Notably, KEAP1 mutational status was associated with early progression (63.6%; n = 7), which is consistent with the poor prognosis often seen for these patients. Conversely, the majority of patients with cell cycle (51.9%; n = 14) and WNT pathway (50%; n = 12) mutations experienced late progression, highlighting an opportunity for combination therapy in this subgroup.
Individual RTK genes showed varying patterns of response. For instance, in patients with EGFR co-mutations (n = 13), 15.4% had early progression, 38.5% had late progression, and 46.2% had ongoing disease control. Yet among those with NTRK co-mutations, 41.7% had early progression, 50.0% had late progression, and 8.3% had ongoing disease control. The majority of patients with FGFR and RET co-mutations had ongoing disease control (60.0% and 75.0%, respectively).
“RTK genes still [had] no association with either early or late progression, and this warrants further investigation, in larger studies of course,” said Skoulidis, an assistant professor in the Department of Thoracic/Head and Neck Medical Oncology at The University of Texas MD Anderson Cancer Center in Houston.
“In view of these results, we hypothesize that baseline tumor genomic profiles may affect clinical response in patterns of resistance to sotorasib,” Skoulidis said.
Further investigation into co-mutational status and sotorasib response is needed, he added, emphasizing that the results should be interpreted with caution and should be considered hypothesis generating.
“The small sample size of each of the subgroups represents a limitation of the study. Nonetheless, groupings of permutations into functional pathways may help determine patterns of response and resistance,” Skoulidis said
REFERENCES:
1. FDA grants accelerated approval to sotorasib for KRAS G12C mutated NSCLC. FDA. May 28, 2021. Accessed September 8, 2021. https://bit.ly/3jqh0Qk
2. Skoulidis F, Li BT, Dy GK, et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384(25):2371-2381. doi:10.1056/NEJMoa2103695
3. Ramalingam SS, Skoulidis F, Govindan R, et al. Efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK 100. Poster presented at: International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract P52.03.
4. Skoulidis F, Schuler M, Wolf J, et. Al. Genomic profiles and potential determinants of response and resistance in KRAS p.G12C-mutated NSCLC treated with sotorasib. Paper presented at: International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract MA14.03.
5. Furuya N, Ito K, Sakaguchi T, et al. The impact of EGFR mutation status and brain metastasis for non-small cell lung cancer treated with ramucirumab plus docetaxel. Oncology. 2020;98(9):661-668. doi:10.1159/000507050
