Extended CLEAR Trial Follow- Up Shows Maintained Efficacy of Lenvatinib/Sunitinib in RCC
Toni K. Choueiri, MD

With an extra 7 months’ median follow-up of patients in the phase 3 CLEAR trial (NCT02811861), efficacy with the combination of lenvatinib (Lenvima) and pembrolizumab (Keytruda) was maintained in patients with advanced renal cell carcinoma (RCC), according to updated findings presented at the 2021 International Kidney Cancer Symposium.1 Although neither the lenvatinib/pembrolizumab arm nor the sunitinib (Sutent) treatment arm reached a median overall survival (OS) in all randomized patients, a trend for improvement was seen with the combination regimen.
“No substantial changes in efficacy end points were observed from the original data cutoff date,” study authors, led by Toni K. Chouieri, MD, of Dana-Farber Cancer Institute in Boston, Massachusetts, wrote in their poster. “Along with the previously observed efficacy benefits with lenvatinib plus pembrolizumab, this follow-up analysis further supports lenvatinib plus pembrolizumab as a first-line treatment for patients with advanced RCC.”
The CLEAR study explored frontline treatment for patients with advanced RCC with a clear cell component. Enrolled patients had a Karnofsky performance status of at least 70 and adequate organ function. These patients were randomized 1:1:1 to receive either lenvatinib 20 mg daily plus pembrolizumab 200 mg intravenously every 3 weeks, lenvatinib 18 mg daily plus everolimus (Afinitor) 5 mg daily, or sunitinib monotherapy 50 mg daily for 4 weeks on and 2 weeks off treatment. The primary end point was progression-free survival (PFS) by independent review committee. Prior cutoff for the initial results of the trial was on August 28, 2020, with a median follow-up of 26.6 months.2
This study led to the FDA approval of lenvatinib and pembrolizumab for the first-line treatment of adult patients with advanced RCC.3 This arm and the standard-of-care comparator arm of sunitinib monotherapy were the focus of the updated analysis, which comprised a median follow-up of 33.7 months for lenvatinib and pembrolizumab with a cutoff date of March 31, 2021.1
Of the 355 patients in the lenvatinib/pembrolizumab arm, the median age was 64 (range, 34-88), 63.9% had intermediate risk by Memorial Sloan Kettering Cancer Center (MSKCC) criteria, 59.2% had intermediate risk by International Metastatic RCC Database Consortium criteria (IMDC), 7.9% had sarcomatoid features, 30.1% had positive PD-L1 expression, and 73.8% had undergone prior nephrectomy. In the sunitinib arm (n = 357), the median age was 61 years (range, 29-82), 63.9% had intermediate risk by MSKCC criteria, 53.8% had intermediate risk by IMDC criteria, 5.9% had sarcomatoid features, 33.3% had positive PD-L1 expression, and 77.0% had undergone prior nephrectomy. As of the updated cutoff date, 32.1% of patients in the lenvatinib/pembrolizumab arm and 13.7% in the sunitinib arm were receiving ongoing treatment.
The median PFS was 22.1 months (95% CI, 18.2-27.7) with lenvatinib and pembrolizumab treatment compared with 9.5 months (95% CI, 7.9-11.1) with sunitinib treatment (HR, 0.47; 95% CI, 0.38-0.57). The study authors noted that PFS was assessed in the analysis only by investigators by RECIST criteria.
Median OS was not estimable in both treatment arms (HR, 0.72; 95% CI, 0.55-0.93). However, the Kaplan-Meier curves did eventually cross.
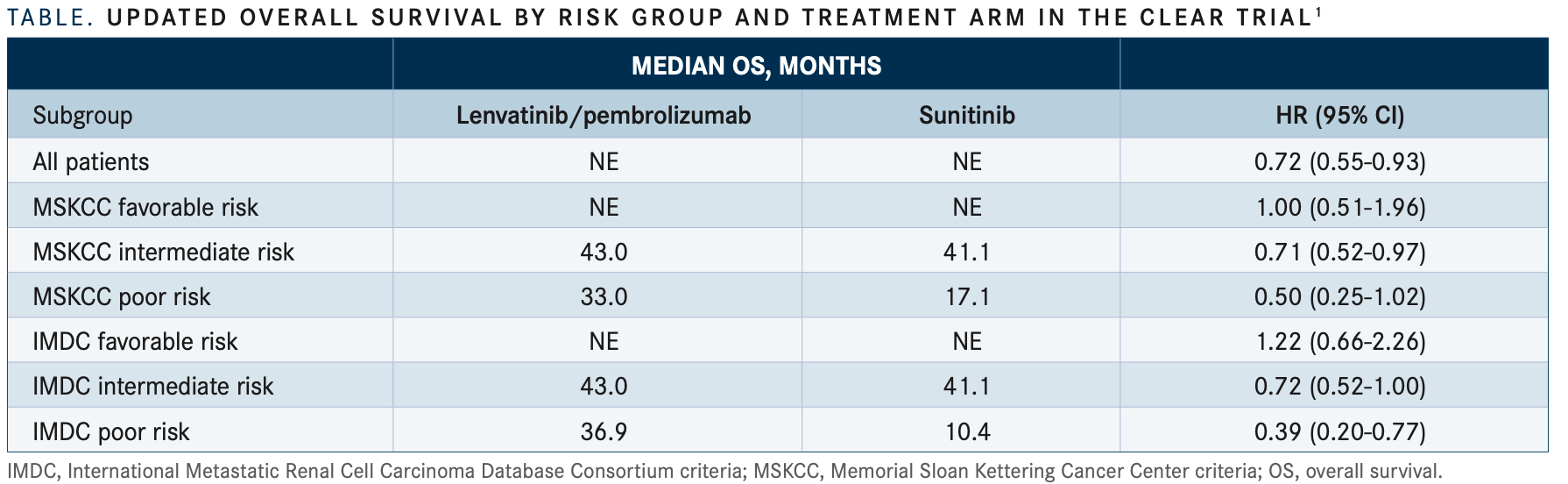
In a subgroup analysis for OS, patients with intermediate or poor risk showed an even greater benefit from lenvatinib/ pembrolizumab over sunitinib treatment compared with those with favorable risk; this was seen with both sets of prognostic risk criteria (TABLE1). However, Choueiri et al did note that the interpretation was limited by the low number of events.
The objective response rate was 68.7% (95% CI, 63.9%- 73.6%) in patients treated with lenvatinib and pembrolizumab compared with 34.5% (95% CI, 29.5%-39.4%) with sunitinib (relative risk, 2.00; 95% CI, 1.70-2.34). In the doublet arm, complete responses (CRs) were seen in 10.4%, partial responses (PRs) in 58.3%, and stable disease (SD) in 20.8%. The median time to response (TTR) was 1.94 months (range, 1.41-20.14) and the median duration of response (DOR) was 27.9 months (95% CI, 22.1-36.8).
In the sunitinib monotherapy arm, CRs were reported in 2.0%, PRs in 32.5%, and SD in 44.3%. The median TTR was 2.00 months (range, 1.51-17.28) and the median DOR was 14.8 months (95% CI, 11.6-17.3).
REFERENCES
1. Choueiri TK, Powles T, Porta C, et al. A phase 3 trial of lenvatinib plus pembrolizumab versus sunitinib as a first-line treatment for patients with advanced renal cell carcinoma: overall survival follow-up analysis (CLEAR study). Presented at: 2021 International Kidney Cancer Symposium; November 5-6, 2021; Austin, TX. Abstract E41.
2. Motzer R, Alekseev B, Rha SY, et al; CLEAR Trial Investigators. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384(14):1289-1300. doi:10.1056/NEJMoa2035716
3. FDA approves lenvatinib plus pembrolizumab for advanced renal cell carcinoma. FDA. Updated August 11, 2021. Accessed December 9, 2021. https://bit.ly/3rZowFY
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen