Expert Explores the Rationale for Liver-Directed Therapies in Metastatic NETs
Management of neuroendocrine liver metastases relies on many treatment modalities, ranging from surgery to ablation. Clinicians have in their armamentarium interventional radiology techniques that use regional and systemic treatments spanning a diverse group of biologic, cytotoxic, and targeted agents.
Juan C. Camacho, MD

Juan C. Camacho, MD
Management of neuroendocrine liver metastases relies on many treatment modalities, ranging from surgery to ablation. Clinicians have in their armamentarium interventional radiology techniques that use regional and systemic treatments spanning a diverse group of biologic, cytotoxic, and targeted agents.
Juan C. Camacho, MD, explained the rationale for using liver-directed therapies and peptide receptor radionuclide therapy (PRRT) in patients with neuroendocrine tumors (NETs) that have metastasized at the 2019 Society of Nuclear Medicine and Medical Imaging Annual Conference, held June 22-25 in Anaheim, California.1
The dominant factor affecting the overall prognosis in patients with NETs remains the presence of liver metastases, said Camacho, assistant attending radiologist in the Department of Radiology of Memorial Sloan Kettering Cancer Center in New York City, during the presentation.
The 5-year survival rate for NETs generally is 47.5%, with rates of 58.1% in patients who have differentiated tumors and 8.1% for those with small cell tumors. If the patient has liver metastases, the 5-year survival is worse, about 30% to 60%, according to Frilling et al.2They reported that the most common primary gastroenteropancreatic NET sites include the small intestine (28%), appendix (20%), pancreas (16%), rectum (15%), and colon (13%), with distant metastases developing predominantly in the small intestine, pancreas, and colon (FIGURE).2
The use of liver-directed therapies in the metastatic settingis particularly beneficial because “only about 20% to 30% of patients are candidates for resection, and the percentage of R0 resections is highly variable, ranging from 38% to 100%,” Camacho said.3Liver- directed therapies include bland embolization, chemoembolization, radioembolization, and PRRT.
Further, a retrospective study by Du et al demonstrated that surgery and nonsurgical locoregional interventions (NSLRIs) both prolonged patient survival, with the differences reaching statistical significance.4The investigators reported that the median overall survival (OS) and survival rate in the surgery group were significantly better than those in the nonsurgery group, which was also true for the comparison between the NSLRI and non-NSLRI groups.
Treatment Algorithms Continue to Confuse
“However, that survival is radically different for patients who receive aggressive locoregional therapy in addition to systemic therapies,” Camacho said, referring to the study’s finding of markedly enhanced median OS among patients who received both NSLRIs and surgery versus surgery alone.Camacho noted that although there is evidence that local therapy adds a survival benefit, the treatment algorithms remain somewhat confusing. In the presence of disease growth, high volume of disease, or clinical symptoms from the disease, patients are started on a somatostatin analog, which is generally well tolerated. However, Camacho said, patients eventually progress over time. Treatment options after progression focus on ischemia, radiation, alkylating agents, and targeted agents.
“Although there are many available treatments in the algorithm, there is no definitive guideline. [Adding to the confusion is that] randomized controlled trials are not available for each of the strategies,” Camacho said.
Locoregional Therapies
Camacho said that guidelines that came out of a recent North American NET-livermetastases consensus conference5 indicate that despite the presence of multiple locoregional therapies throughout the algorithm, there is insufficient evidence to reach a consensus regarding their relative advantages. European guidelines exhibit the same vagueness, said Camacho. “We know some of these locoregional therapies actually work, but we don’t know exactly how to use them.”In phase II trials involving ablation therapy, overall 5-year survival rates ranged from 37% to 57%. The most favorable results were seen in patients with limited hepatic tumors (˂5 lesions) and dominant lesions measuring˂5 cm with postinterventional margins of˃1 cm. “This is another technique used in cytoreduction,” Camacho said of ablation therapy.
When comparing intra-arterial therapy (IAT) with PRRT, Camacho said that he did not consider PRRT a “direct competitor with IAT. The population that benefits the most from PRRT is different from the population that benefits from IAT.”
In IAT, predictors of response or survival include low hepatic tumor burden (˂50%), low-grade tumor differentiation, and absence of extrahepatic disease. Candidates for PRRT have grade 1/2 tumors, hepatic and extrahepatic metastases ˂50%, and detectable somatostatin receptor based imaging.
“Also, it is very well described that liver metastases do not necessarily respond to PRRT; therefore, liver-directed therapies could be used as an adjuvant therapy in those patients who are undergoing PRRT,” Camacho said.
In studies comparing bland embolization and transarterial chemoembolization (TACE), outcomes seem to slightly favor TACE in terms of both radiological response and OS.5
“However, the differences between the 2 do not appear to be statistically significant,” Camacho noted, adding that he recommends basing treatment decisions on the toxicity profile.
Yttrium 90 (90Y) offers excellent outcomes with significant objective response rates and median survival of up to 70 months. Braatetal, 90Y demonstrated a high disease control rate of 91% in patients with progressive disease and alleviation of neuroendocrine neoplasmrelated symptoms in 79% of symptomatic patients.6
The combination of PRRT with 90Y is reserved for refractory patients with liver metastases. “90Y does not appear to have toxicity when given following PRRT; however, the limited data suggest that patients treated with PRRT after 90Y did not benefit from the therapy as much [as patients treated in the opposite order],” Camacho said. “This is probably due to loss of receptors or dedifferentiation of the tumors.”
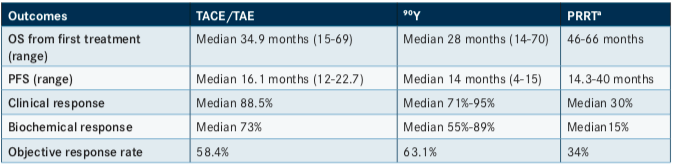
Camacho said that median progression-free survival (PFS) and OS were relatively comparable between TACE/transarterial embolization (TAE), 90Y, and PRRT. However, he noted a difference in median clinical, biochemical, and objective response rates, suggesting a disadvantage of PRRT (albeit one without proven statistical significance or patient benefit) (TABLE).1He added that the PRRT data were compiled before the NETTER-1 trial,7which demonstrated that treatment with 177Lu-Dotatate resulted in markedly longer PFS and a significantly higher response rate than high-dose, long-acting, repeatable octreotide (LAR) among patients with advanced midgut NETs.
Future Directions
Toxicity for each modality varied, with postembolization syndrome associated with embolization and TACE, myelodysplastic syndrome seen in PRRT, myelosuppression observed with alkylating agents, and adverse effects associated with targeted agents.In data presented by Ziv et al,851 patients with neuroendocrine liver metastases who had under- gone TAE and had available mutational analysis results were identified. The investigators noted that a mutation in theDAXXgene was associated with reductions in hepatic PFS and time to hepatic progression after TAE, findings that were independent of tumor grade.
Further, in patients who received alkylating agents, Camacho observed tumor mutation burden increasing over time. “This could possibly transform the disease into a more aggressive form. This should be investigated further,” said Camacho.
He noted that promising approaches currently being explored include combination therapies and the use of intra-arterial PRRT.
References
- Camacho J. How to use liver directed therapy and PRRT in patients with liver dominant disease. Presented at: Society of Nuclear Medicine and Molecular Imaging 2019 Annual Meeting; June 22-25, 2019; Ana- heim, CA. Abstract CE57. bit.ly/2SpvZKU
- Frilling A, Modlin IM, Kidd M, et al; Working Group on Neuroendo- crine Liver Metastases. Recommendations for management of patients with neuroendocrine liver metastases.Lancet Oncol. 2014;15(1):e8-21. doi: 10.1016/S1470-2045(13)70362-0.
- Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases.Cancer. 2015;121(8):1172-1186. doi: 10.1002/cncr.28760. 4. Du S, Ni J, Weng L, et al. Aggressive locoregional treatment improves the outcome of liver metastases from grade 3 gastroenteropancreatic neuroendocrine tumors.Medicine. 2015;94(34):e1429. doi: 10.1097/ MD.0000000000001429.
- Du S, Ni J, Weng L, et al. Aggressive locoregional treatment improves the outcome of liver metastases from grade 3 gastroenteropancreatic neuroendocrine tumors.Medicine. 2015;94(34):e1429. doi: 10.1097/ MD.0000000000001429.
- Kennedy A, Bester L, Salem R, Sharma RA, Parks RW, Ruszniewski P; NET-Liver-Metastases Consensus Conference. Role of hepatic intra- arterial therapies in metastatic neuroendocrine tumours (NET): guide- lines from the NET-Liver-Metastases Consensus Conference.HPB(Oxford). 2015;17(1):29-37. doi: 10.1111/hpb.12326.
- Braat AJAT, Kappadath SC, Ahmadzadehfar H, et al. Radioembolization with 90Y resin microspheres of neuroendocrine liver metastases: inter- nationalmulticenterstudyonefficacyandtoxicity.CardiovascIntervent Radiol. 2019.42(3): 413-425. doi: 10.1007/s00270-018-2148-0.
- Strosberg J, El-Haddad G, Wolin E, et al; NETTER-1 Trial Investigators. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors.N Engl J Med. 2017;376(2):125-135. doi: 10.1056/NEJMoa1607427.
- . Ziv E, Rice SL, Filtes J, et al.DAXXmutation status of emboliza- tion-treated neuroendocrine tumors predicts shorter time to hepatic pro- gression.J Vasc Interv Radiol. 2018;29(11):1519-1526. doi: 10.1016/j. jvir.2018.05.023.
