Epigenetic Targeted Agent May Combat Resistance in Many Cancers
The phase I results of RRx-001, which has multiple epigenetic targets, have been published in Lancet Oncology. The drug is of interest because epigenetic alterations are associated with resistance to chemotherapy.
Lancet Oncology.1The drug is of interest because epigenetic alterations are associated with resistance to chemotherapy. Drugs that target these alterations are a promising avenue for research.
RRx-001 had antitumor properties in several tumor types and, preliminary evidence was obtained that it can chemosensitize and resensitize tumors to subsequent therapies. Thus, it may have a role in overcoming acquired resistance.
“The ability to provide resensitization to previous chemotherapy would be a change in [the] treatment framework, allowing for repetitive reintroduction of existing lines of therapy, interspersed at designated intervals with the priming or episensitizing drug,” wrote Reid et al.1
RRX-001 is a member of a new class of compounds, the dinitroazetidines, and sourced from the defense and aerospace industry. RRx-001 binds to hemoglobin and alters the properties of a subpopulation of erythrocytes. These erythrocytes then adhere to the tumor endothelium, blocking tumor microvasculature. This creates hypoxic conditions leading to the production of reactive oxygen and nitrogen species, which can epigenetically modulate DNA methylation, histone deacetylation and lysine demethylation. Preclinical studies on cell lines showed that it inhibits proliferation, and the generation of reactive oxygen and nitrogen species, causes DNA damage, and triggers caspase-independent apoptosis. It has been shown to inhibit tumor growth and affect tumor vasculature in animal models.1-4
The primary objective of the phase I open label study was to evaluate the safety tolerability and dose-limiting adverse events (AEs) of multiple increasing doses of intravenously delivered RRx-001, in order to quantify single-dose pharmacokinetics and discover a suitable dose for a phase II trial. Determining any antitumor activity of the drug by RECIST assessment was a secondary objective.1
The study enrolled 25 patients with advanced malignant incurable tumors that were rapidly progressing. The majority of the patients were male (60%), and the most common tumor type was colorectal cancer (CRC; 44%), followed by head and neck (16%), and pancreatic (12%). Other tumor types included lung, ovarian, cholangiocarcinoma, melanoma and oligodendroglioma. There were six dose cohorts, ranging from 16.7 mg/m2to 83 mg/m2, and patients were treated once or twice a week for a minimum of 4 weeks.
More than 44% of patients had received >3 previous regimens, while others had previously recieved 1 to 3 previous treatments. Ninety-two percent of patients had former treatment with chemotherapy, 80% surgery, and 48% radiation therapy. Tumor size was measured at baseline (<2 weeks before treatment began) and then every 4 or 8 weeks until progression using CT, PET-CT or MRI.1
Safety of RRx-001
The majority of AEs were grade 1 and 2; the most common being treatment-related injection site pain and forearm vasodilation, which in most cases resolved in a few minutes when infusion was stopped. To reduce the pain, patients were infused over a duration of 8 hours, and at the highest dose, some patients were infused twice weekly. Analgesics, nonsteroidal antiinflammatory drugs or corticosteroids were prescribed as needed. The authors reported no treatment-related deaths and no dose limiting toxicities in any dose cohort. Also, the maximum tolerated dose was not reached. Treatment compliance was described as very good.1
Antitumor Activity of RRx-001
Patients’ responses to treatment (21 evaluable) at 8 weeks are shown in the figure. Disease control was evident at first assessment in 71% of patients. Stable disease was experienced for more than 4 months in 28% of patients, and this was independent of the dose received.1
Figure: Percentage of patients responses.
Figure: Percentage of patients responses.
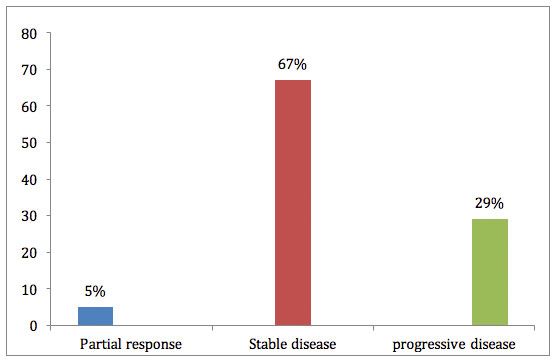
Median overall survival (OS) for all patients was 8.2 months (95% CI, 4.7-18.8), and median follow-up was 4.2 months. During effective treatment, there were no new metastatic foci, and tumors exhibited pseudo enlargement and central necrosis. Tumor density dropped by more than 10% in 36% of 22 lesions, without a change in tumor measurement. The authors state that this reduction suggests a “cytolytic rather than cytostatic response.”1
It was expected that as an epigenetic therapy, RRx-001 would sensitize the tumors to subsequent therapy. Ten out of 12 patients from all dose cohorts went on to radiotherapy and chemotherapy and responded well with a median survival of 20.8 months (95% CI, 15.6 not reached).1
Evidence that RRx-001 induced resensitization was obtained in four patients, who were resistant to chemotherapy and for whom there were no further treatment options. These patients were rechallenged with previously effective chemotherapies (3 CRC with FOLFIRI, 1 lung cancer with gemcitabine). These patients responded to the treatments, and their median OS was 17.8 months (95% CI, 10.0-not reached).1
RRx-001 and the Future
The favorable safety profile of RRx-001, evidence of antitumor activity and importantly of chemo sensitization, and resensitization predict clinical utility of this drug. It is being further investigated in a phase II open-label randomized trial versus regorafenib in subjects with metastatic CRC (NCT 02096354); the primary outcome measure is OS. One of the secondary outcome measures is the response of the patients to subsequent therapies, to test for resensitization.1
Concluding their discussion, the authors stated that, “Additionally because epigenetic agents can affect immunological mechanisms, RRX-001 has the potential to enhance the activity of immune checkpoint inhibitors in combination,” and added that, “The priming potential of RRx-001 both as a single drug and in combination with radiation, immunotherapy, and chemotherapy will be explored in future studies.”1
References
1. Reid T, Oronsky B, Scicinski J, et al. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalationphase 1 study.Lancet Oncol. 2015 Aug 18. pii: S1470-2045(15)00089-3. doi:10.1016/S1470-2045(15)00089-3. [Epub ahead of print]
2. Ning S, Bednarski M, Oronsky B, et al. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials.Cancer Res. 2012;72:2600-2608.
3. Cabrales P, Reid T, Oronsky B et al. RRx-001 an EXO-based epigenetic anti-cancer agent in phase 2 clinical trials. ISEV2014 Educational Event; San Diego, CA, USA; Oct 26, 2014
4. Hongjuan Zhao, Shoucheng Ning, Jan Scicinski et al. RRx-001: A double action systemically non-toxic epigenetic agent for cancer therapy. American Association for Cancer Research annual meeting Philadelphia 2015. Abstract 3515