Early Activity Seen With Selective RET Inhibitors in RET-Altered Solid Tumors
Two selective inhibitors of the rearranged during transfection kinase have shown early efficacy in phase I studies of patients with various solid tumors harboring RET alterations, offering a potential new treatment option for patients with RET-altered tumors with reduced toxicity.
Vivek Subbiah, MD
Two selective inhibitors of the rearranged during transfection (RET) kinase have shown early efficacy in phase I studies of patients with various solid tumors harboringRETalterations, offering a potential new treatment option for patients withRET-altered tumors with reduced toxicity.
“There is a critical unmet need for effective drugs against cancers that have theRETalteration, as there are no highly potent inhibitors currently approved specifically for theseRET-driven cancers,” Vivek Subbiah, MD, assistant professor in the Department of Investigational Cancer Therapeutics, Division of Cancer Medicine at The University of Texas MD Anderson Cancer Center, said in a statement. “The current treatments for these cancers are limited to traditional chemotherapy and earlier generations of multiple kinase inhibitors. These options have had limited success with often considerable side effects that significantly impact the patient’s quality of life.”
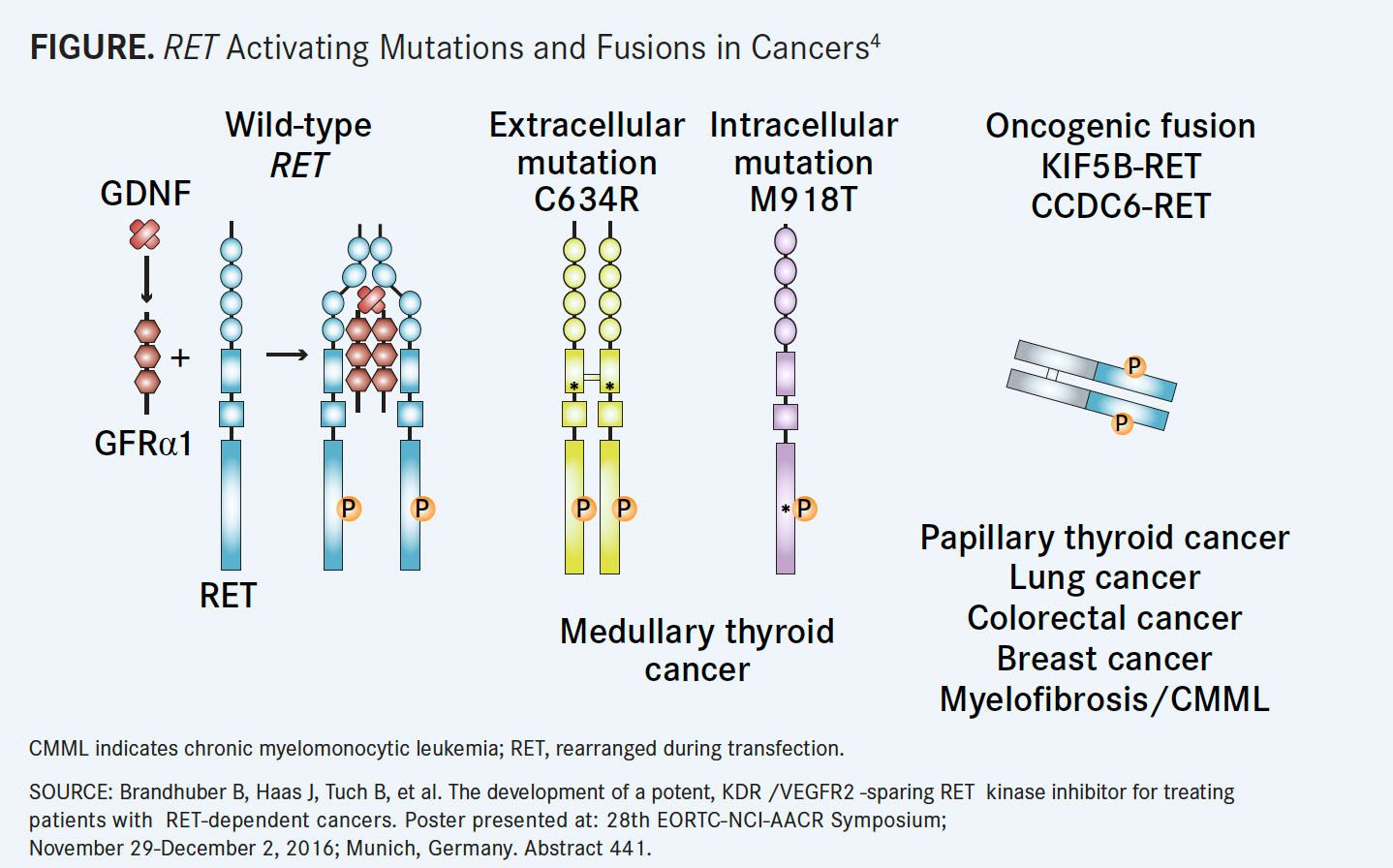
RET is a single-pass transmembrane receptor tyrosine kinase that is vital for the normal development of several tissues and cell types, particularly of neural and genitourinary tissues, and for homeostasis.REThas been implicated as a driver of several tumor types.1
RET-activating point mutations are commonly found in medullary thyroid cancers (MTCs), amounting to more than 60% of MTC cases. Alternatively,RETgene rearrangements can be found in more than 10% of papillary thyroid tumors (PTCs), 1% to 2% of nonsmall cell lung cancers (NSCLCs), and 1% of breast, colorectal, pancreatic, and esophageal cancers.REThas also been identified as a driver in some melanomas and chronic myelomonocytic leukemias.2
HeritableRETmutations can be found in patients with multiple endocrine neoplasia type 2, a cancer predisposition syndrome that is often characterized by early onset MTC.1,3Point mutations inRETare typically found in C634W, M918T, or V804L/M.
Common somatic rearrangements includeRETfusions withKIF5Bwhich can be found in 1% to 2% of lung adenocarcinomas,CCDC6,NCOA4, andTRIM33all of which are typically found in PTCs, but also in few cases of NSCLC and colorectal cancers (FIGURE4).
No approved therapies currently targetRETmutations and alterations selectively.
“Precision oncology has benefitted patients withEGFR,ALK, andROS1aberrations. Unfortunately, patients withRET-altered cancers have not benefitted from precision oncology targets to date,” said Subbiah, who is also the associate medical director of the Clinical Center for Targeted Therapy. “There was a need to design a selective drug for the RET pathway.”
Previous therapies for patients with RET alterations often consisted of multikinase inhibitors such as cabozantinib (Cabometyx) and vandetanib (Caprelsa). Both of these agents have been approved for the treatment of patients with metastatic or locally advanced MTC and have proven efficacy inRETfusiondriven NSCLC. However, multikinase inhibitors are often associated with significant off-target toxicity and neither agent has shown activity with theRETV804L/M mutations.2
BLU-667
A phase I study of the highly potent and selective oral inhibitor BLU- 667, which is currently in development by manufacturer Blueprint Medicines Corporation, demonstrated early efficacy in targeting oncogenicRETfusions, point mutations, and resistance mutations.
Because BLU-667 is much more selective for RET over other kinases and has proved effective in stopping genetic mutations that are related to resistance to multikinase therapy, BLU-667 was selected for this investigation, according to a press release issued by MD Anderson.5The agent demonstrated increased potency in inhibiting the kinase activity of wild-typeRETand activatedRETmutations and fusion variations compared with the multikinase inhibitors cabozantinib, vandetanib, and RXDX-105.2
In the open-label, first-in-human phase I ARROW trial, the findings of which were presented by Subbiah at the 2018 American Association of Cancer Research Annual Meeting, the investigators aimed to define the maximum tolerated dose (MTD) of BLU-667, as well as the safety, pharmacokinetics (PKs), and antitumor activity in patients with advancedRET-altered solid tumors.6
Fifty-one patients were enrolled with unresectable advanced solid tumors. Twenty-nine patients were found to haveRET-mutant MTC; 19, NSCLC withRETfusions; 2, PTC; and 1, paraganglioma. There was a median of 1 prior anti-neoplastic therapy (range, 0-8) among the patients in the trial.
Patients were given BLU-667 orally at doses ranging from 30 to 400 mg daily on a 4-week cycle following a Bayesian Optimal Interval design. This allowed for additional accrual to dose levels declared safe. The MTD was not reached in this trial.
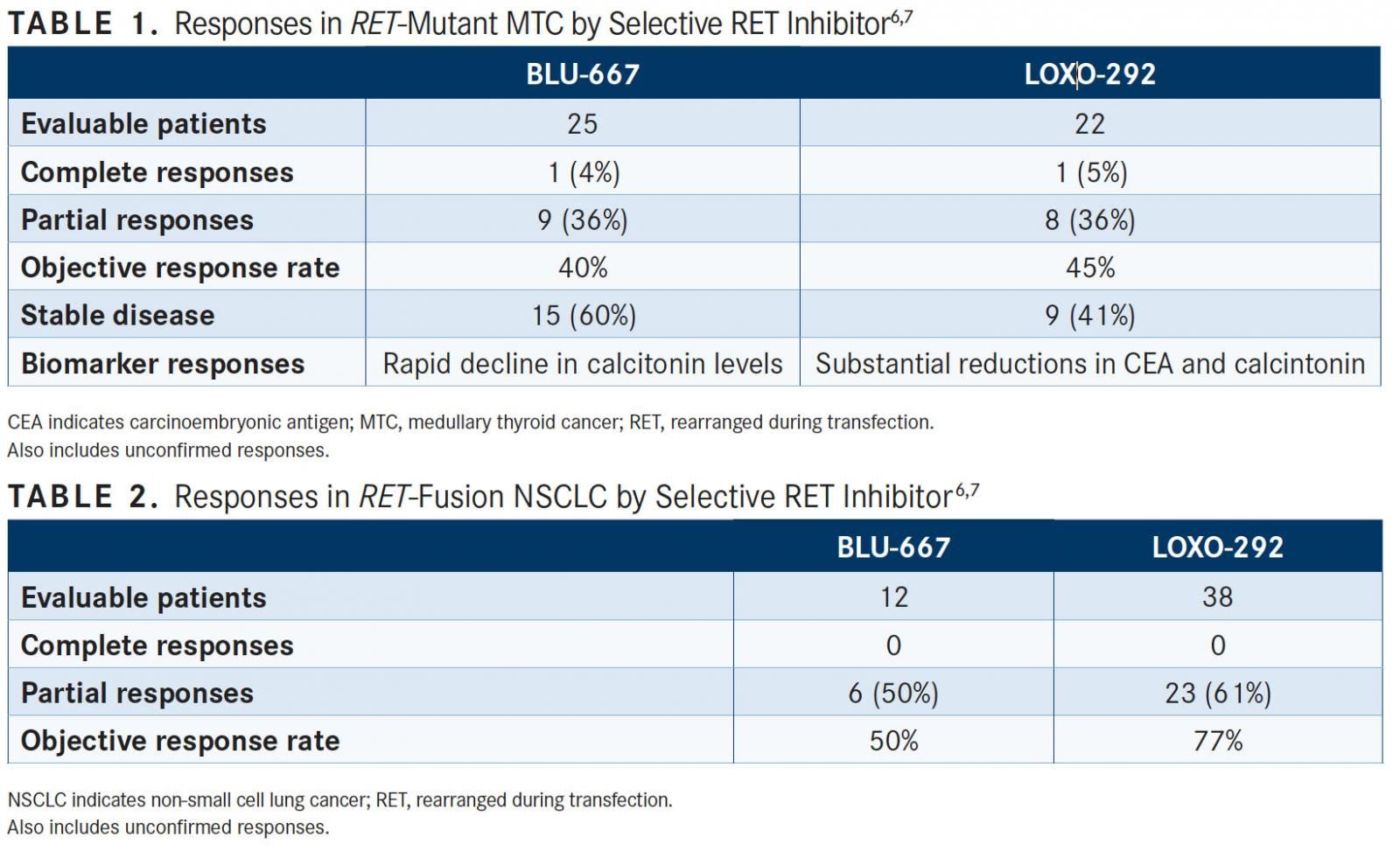
Broad antitumor activity was found with this agent, showing a best objective response rate (ORR) of 37% (95% CI, 20%-56%) in patients withRETalterations who had received doses of ≥60mg and had at least 1 postbaseline response assessment (n = 30). Patients with NSCLC and MTC had best ORRs of 50% and 40%, respectively (TABLES 1 and 2). Among those patients withRETfusions and mutations, there was an ORR of 45%.
Overall, there were 40 evaluable patients, of which 1 had a complete response, 17 achieved partial responses, 20 had stable disease, and 2 had progressive disease. Subbiah said that these results are encouraging due to the broad antitumor activity seen regardless of tumor type; genomic aberration; fusion mutation; prior therapies that included immunotherapy, chemotherapy, and multikinase targeted therapy; and number of prior therapies in patients.
A rapid decline in blood and tumor biomarkers was found to be associated with antitumor activity, while the PKs showed rapid BLU-667 absorption (Tmax2-4 hours), long half-life (>12 hours), and exposure (area under the curve and Cmax) in the expected therapeutic range based on tumor xenograft models.
Overall, BLU-667 appeared to be well tolerated amongst the patients. The most common adverse event (AE) was grade 1 constipation (23%). Grade 3 to 4 AEs were also found, including hypertension (8%) and neutropenia (4%). Additional AEs included increased alanine aminotransferase, fatigue, and diarrhea and a decrease in white blood cells (2% each). Researchers recorded 3 dose-limiting toxicities (DLTs). There were no grade 4/5 AEs.
After the data cutoff, 41 of 51RET-altered patients remained on the BLU-667 treatment. Subbiah said that this was fantastic for a phase I study since patients had been receiving the drug in lower doses. A dose-escalation portion of this trial is currently enrolling globally.
Subbiah also noted in an interview withTargeted Therapies in Oncologythat the investigators are looking for patients who may develop resistance mutations. “We are doing plasma cell-free DNA [testing] and collecting them at various time points and actively looking at what would be the mechanism of resistance,” he commented.
LOXO-292
Another potent selective new RET inhibitor has demonstrated promising antitumor activity in patients with advancedRET-altered solid tumors, according to findings from the ongoing phase I LIBRETTO-001 trial of LOXO-292 that was presented at the 2018 ASCO Annual Meeting.7Encouraging results were also seen in patients who have been previously treated with multikinase inhibitors and patients with brain metastases.
According to Loxo Oncology, the company developing LOXO-292, the agent was designed to inhibit both native RET signaling and potential acquired resistance mutations. The investigational agent has shown preclinical activity against activatingRETmutations and fusions,4potential resistance mutations, and brain metastases.
At the April 2018 data cutoff, 82 patients had been treated across 7 cohorts of LOXO-292, with doses ranging from 20 mg every day to 240 mg twice daily. The study enrolled patients withRETfusion‒positive cancer or those withRETmutations. TheRETfusion group (n = 49) included 38 patients with NSCLC, 9 with papillary thyroid cancer, and 2 with pancreatic cancer. TheRET-mutated arm consisted solely of patients with MTC (n = 29).
The median age of patients in the trial was 61 years (range, 17-88) and the primary ECOG performance status score was 71%. The median number of prior therapies was 3 (range, 1-9). Nearly a third of patients received ≥2 prior multikinase inhibitors (29%), with 66% of patients receiving at least 1 of these agents prior to study entry. Prior immunotherapy was received by 24% of patients and brain metastases were present for 15% of patients.
For patients withRETfusion-positive NSCLC (n = 38), there were 20 partial responses (PRs) and 3 PRs that were still awaiting confirmation on subsequent scans (ORR, 77%; 95% CI, 58%-90%) (TABLE 2). Four patients had stable disease and 3 were not yet evaluable. As of the cutoff date, no patients with NSCLC had developed progressive disease, with 90% remaining on treatment with LOXO-292. Moreover, all patients (n = 3) with measurable intracranial lesions responded to LOXO-292.
“All 12 patients with CNS metastases at baseline remained on treatment,” added lead investigator Alexander E. Drilon, MD, a medical oncologist at the Memorial Sloan Kettering Cancer Center.
For those with MTC, responses were also observed regardless of starting dose and mutation type. Moreover, there was a substantial decline in carcinoembryonic antigen (CEA) and calcitonin levels following treatment with LOXO-292 (TABLE 1). “The majority of MTC patients also experienced substantial stable reductions in the cancer tumor markersCEA and calcitonin—together with improvement in tumor-related symptoms, like diarrhea and flushing,” said Drilon.
Across all patients withRETfusion‒positive cancer, the ORR was 77% (95% CI, 61%-89%), all of which were partial responses. Six patients had stable disease.
Responses to LOXO-292 were seen acrossRETfusion partners for patients with NSCLC. In those with the most common partner, KIF5B (n = 16), the ORR was 81%. In those with non-KIF5B partners, the ORR was 82%. Additionally, responses were observed regardless of the starting dose and prior therapy.
“In response to LOXO-292, disease regression was observed in the vast majority of patients. This activity did not differ by cancer type, in this patient population composed of those with lung, thyroid, and pancreatic cancer,” Drilon said.
Most treatment-emergent AEs (TEAEs) in the study were grade 1 in severity. The most common events were fatigue (20%), diarrhea (16%), constipation (15%), dry mouth (12%), nausea (12%), and dyspnea (9%). The most common treatment-related AEs were fatigue (13%), dry mouth (6%), nausea (5%), diarrhea (2%), and constipation (2%).
There were only 2 treatment-related grade 3 AEs and no grade 4 events observed in the study. The 2 grade 3 events were tumor lysis syndrome and increased ALT levels, but both events resolved, Drilon noted. “Tumor lysis syndrome represented the only dose-limiting toxicity in the trial,” he said. The MTD had not yet been reached by the April data cutoff.
Following these positive findings, rapid development of the study is planned. The full estimated enrollment for the trial is 180 patients and the primary completion date is August 2019 (NCT03157128).
References:
- Mulligan LM. RET revisited: expanding the oncogenic portfolio.Nat Rev Cancer.2014;14(3):173-186. doi: 10.1038/nrc3680.
- Subbiah V, Gainor JF, Rahal R, et al. Precision targeted therapy with BLU-667 for RET-driven cancers [published online April 15, 2018].Cancer Discov.doi: 10.1158/2159-8290.CD-18-0338.
- Mulligan LM. Progress and potential impact of RET kinase targeting in cancer.Expert Rev Proteomics.2016;13(7):631-633. doi: 10.1080/14789450.2016.1205491.
- Brandhuber B, Haas J, Tuch B, et al. The development of a potent, KDR /VEGFR2 -sparing RET kinase inhibitor for treating patients with RET-dependent cancers. Poster presented at: 28th EORTC-NCI-AACR Symposium; November 29-December 2, 2016; Munich, Germany. Abstract 441.
- First-in-human clinical trial of new targeted therapy drug reports promising responses for multiple cancers [press release]. Houston, TX: MD Anderson; April 15, 2018. mdanderson.org/newsroom/2018/04/first-in-human-clinical-trial-of-new-targeted-therapy-drug-reports-promising-responses-for-multiple-cancers. html. Accessed May 15, 2018.
- Subbiah V, Taylor M, Lin J, et al. Highly potent and selective RET inhibitor, BLU-667, achieves proof of concept in a phase I study of advanced, RET-altered solid tumors. Presented at: 2018 AACR Annual Meeting; April 14-18, 2018; Chicago, IL. Abstract CT043. abstractsonline.com/pp8/#!/4562/presentation/11125.
- Drilon AE, Subbiah V, Oxnard GR, et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers.J Clin Oncol.2018;36(suppl; abstr 102). meetinglibrary.asco.org/record/161573/abstract.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More