Durable Responses Continue for Axi-cel in NHL
After 3 years of follow-up in the ZUMA-5 trial, patients with relapsed/refractory indolent non-Hodgkin lymphoma treated with axicabtagene ciloleucel had durable responses.
Ibrahim Yakoub-Agha, MD, PhD
Head
Hematopoietic Stem Cell Transplantation Unit
Professor of Hematology
Department of Hematology
Lille Faculty of Medicine
Lille University Hospital
Hauts-de-France, France

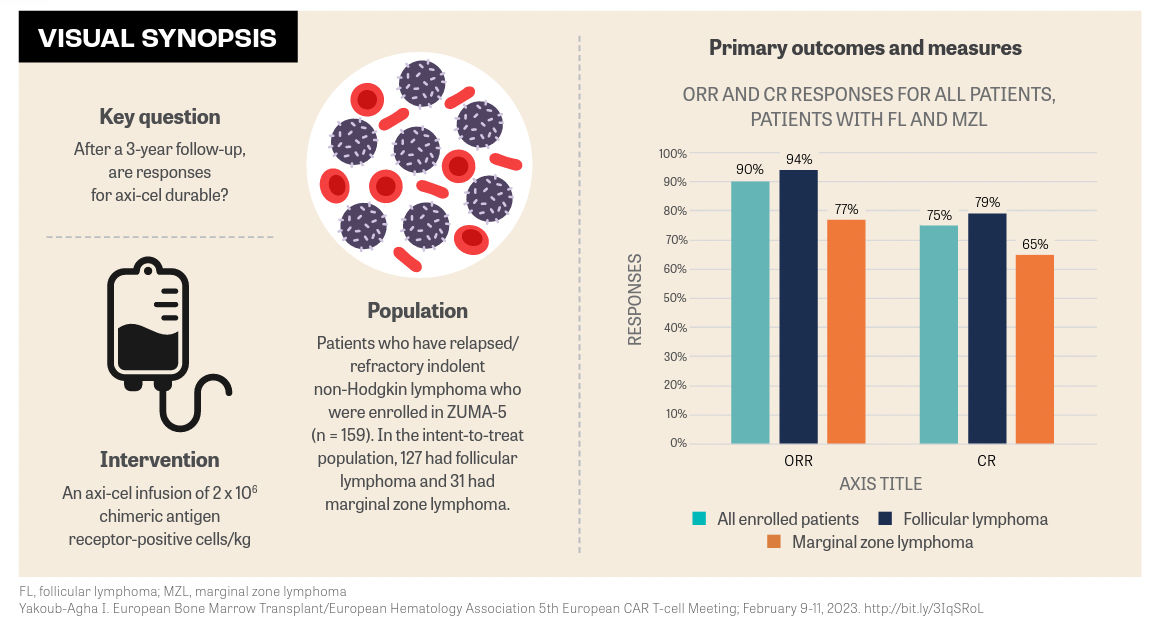
Axicabtagene ciloleucel (Yescarta) continued delivering durable responses after 3 years of follow-up in the ZUMA-5 trial (NCT03105336) in patients with relapsed/refractory (R/R) indolent non-Hodgkin lymphoma (iNHL). The benefits of this extended follow-up were observed in patients with follicular lymphoma (FL) and marginal zone lymphoma (MZL).
After more than 36 months, median follow-up for all enrolled patients with iNHL was 40.5 months (range, 8.3-57.4). Among patients with FL, the median follow-up was 41.7 months (range, 32.7-57.4) and 38 months (range, 8.3-52.3) among patients with MZL, said Ibrahim Yakoub-Agha, MD, PhD, head, Hematopoietic Stem Cell Transplantation Unit, and professor of medicine at Lille University Hospital, Hauts-de-France, France, during a presentation of the data at the European Bone Marrow Transplant/European Hematology Association 5th European CAR T-cell Meeting.1
“Efficacy outcomes were assessed and reported for all 159 enrolled patients,” Yakoub-Agha said. “The overall response rate [ORR] was 90% [95% CI, 84%-94%],” he said. The complete remission (CR) rate was 75%. Patients with FL had an ORR of 94% and a 79% CR rate. Patients with MZL had a 77% ORR and a 65% CR rate.
ZUMA-5 is a phase 2, multicenter, single-arm study evaluating axi-cel, a CD19-directed, autologous T-cell immunotherapy with a CD28 costimulatory domain. In the previous 2-year analysis, after a median follow-up of 30.9 months in patients with FL, the ORR was 94% with a 79% CR rate and 83% in patients with MZL with a 63% CR rate.2
At the data cutoff of March 31, 2022, responses were ongoing in 52% of enrolled patients with iNHL. Responses in patients with FL were 53% and 52% in patients with MZL. Yakoub-Agha noted that median duration of response was not yet reached in enrolled patients with a CR and was 4.9 months in those patients with a partial response (PR).
Median progression-free survival (PFS) also increased with the extended follow-up. In patients with FL, it was 40.2 months and in patients with MZL, it was not reached. Overall survival (OS) was also not yet reached, reported Yakoub-Agha. After 2 years, 2 progression events and 10 deaths had occurred. One patient with MZL died because of disease progression and 4 patients died after subsequent therapy, including 2 with FL who received subsequent allogeneic stem cell transplant.
Looking at key FL subgroups, investigators reported that the estimated PFS at 36 months was consistent in patients with FL regardless of high-risk baseline characteristics. Further, median PFS among patients with FL who had progression of disease within 2 years was consistent with that of all enrolled patients.
“In lymphoma-specific PFS and survival end points, medians have not yet been reached,” said Yakoub-Agha. In the study, 14 deaths were lymphoma-specific with 11 caused by complications of underlying lymphoma and 3 caused by adverse events (AEs) related to study treatment in patients with FL.
These include 1 case of pneumonia, 1 case of organ failure related to cytokine release syndrome, and 1 case of progressive multifocal leukoencephalopathy.
Since the 2-year analysis, AEs of interest were observed only in recently enrolled patients with MZL and no new cases of grade 3 or higher hypogammaglobulinemia after the data cutoff were observed. Secondary malignancies, tumor lysis syndrome, or radiologic complete response were not observed at any time during the study.
The top 5 covariates associated with negative PFS in patients with FL were Follicular Lymphoma International Prognostic Index score and elevated levels of CCL17, CCL22, TNF-α, and IL-16, according to multivariate analysis. Conversely, positive ongoing response and PFS in patients with FL were associated with peak chimeric antigen receptor T-cell levels in blood and total number of infused naïve and central memory T cells.
“After 3 years of follow-up in ZUMA-5, axicel demonstrated continued responses in patients with relapsed/refractory indolent NHL. These data further support axi-cel as a highly effective therapeutic approach for these patients,” Yakoub-Agha concluded.
REFERENCES
1. Yakoub-Agha I. ZUMA-5 3 year follow-up: axi-cell in patients with R/R iNHL. Presented at: European Bone Marrow Transplant/European Hematology Association 5th European CAR T-cell Meeting; February 9-11, 2023; Rotterdam, Netherlands. Accessed February 16, 2023. http://bit.ly/3IqSRoL
2. Neelapu SS, Chavez J, Sehgal AR, et al. 3-year follow-up analysis of zuma-5: a phase 2 study of axicabtagene ciloleucel (Axi-Cel) in patients with relapsed/refractory (r/r) indolent non-Hodgkin lymphoma (iNHL). Presented at: 64th American Society of Hematology Annual Meeting and Exposition; December 10-13, 2022; New Orleans, LA. Abstract 93.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More