Biomarkers in Renal Cell Carcinoma: Present Utility and Future Possibility
Wenxin (Vincent) Xu, MD, provides insight on biomarkers in renal cell carcinoma.

With a richer understanding of renal cell carcinoma (RCC) tumor biology and the addition of immune checkpoint inhibitors (ICIs) to the treatment landscape, the prognosis for many patients with metastatic disease has improved. Still, resistance to checkpoint inhibition is common and contributes to the poor 5-year survival rate of 10% in the metastatic RCC setting.1 Consequently, regimens combining 2 ICIs or an ICI plus a different class of agent have been developed, with some encouraging results. However, adding a second drug increases both the expense of the treatment regimen and the risk of adverse events, inspiring an ongoing search for biomarkers that predict response to ICIs in metastatic RCC.1 These biomarkers would aid in selecting among existing regimens for patients across lines of treatment. Several have been investigated, including polybromo 1 (PBRM1) mutations and T-cell immunoglobin and mucin domain-3 (TIM3) expression, which have shown variable or unreliable results.1,2 Similarly, tumor mutational burden has shown unreliability as a potential predictive biomarker in metastatic RCC.2
When Wenxin (Vincent) Xu, MD, an oncologist at the Lank Center for Genitourinary Oncology at Dana-Farber Cancer Institute in Boston, Massachusetts, spoke about biomarkers in RCC with Targeted Therapies in Oncology™, he began by differentiating between the prognostic, predictive, and treatment-specific uses of biomarkers, pointing out that sometimes there is an overlap. As far as what one might find when conducting an online search for biomarkers in RCC, he said, “you will get hundreds, and while they might be scientifi cally interesting, they’ll most likely never reach the point where a clinician needs to know about them.”
Prognostic Tools
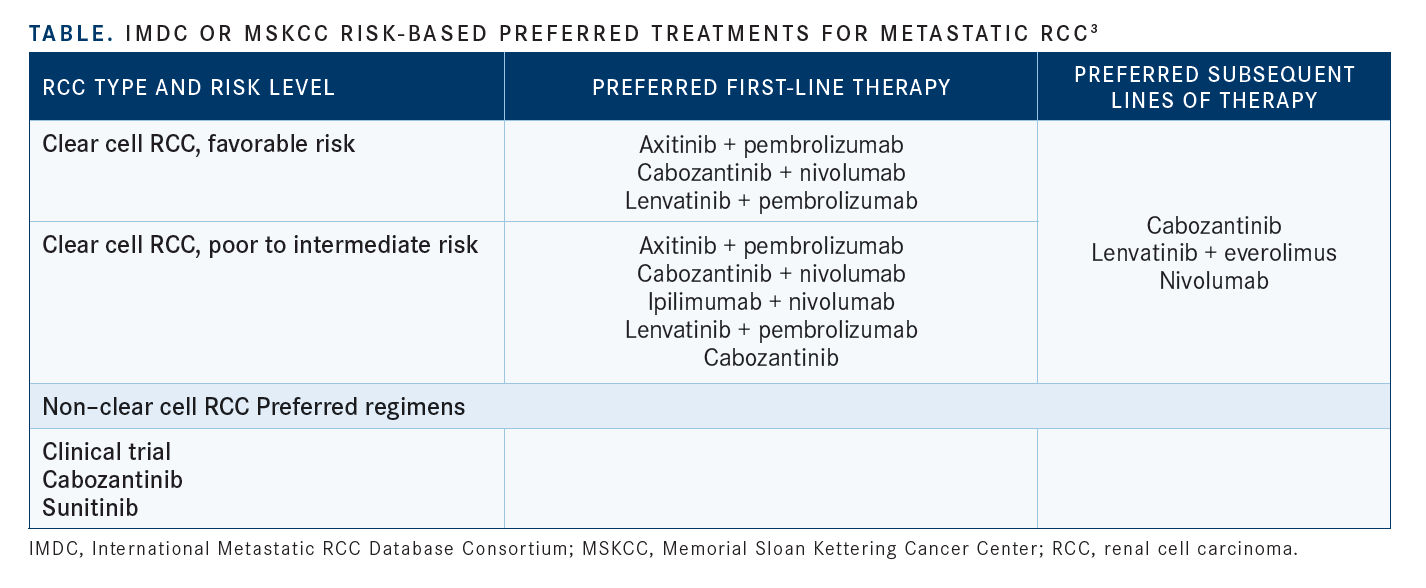
In terms of clinically applicable prognostic tools, risk stratification using a validated risk model, either the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) risk model or the Memorial Sloan Kettering Cancer Center (MSK) risk model, remains central to patient evaluation and treatment decision-making (see TABLE3).3,4 Interestingly, a retrospective study found that disagreement between the 2 risk models was associated with worse patient prognosis when compared with model agreement.5
“In 2022, risk stratifi cation, either with the IMDC or the MSK criteria, is the most important thing that we can do for patients. For example, ipilimumab [Yervoy] plus nivolumab [Opdivo] in the first line is only approved for intermediate- to poor-risk patients. And that bears out the data where the response rates are lower for favorable- risk patients. Whereas the other combinations of tyrosine kinase inhibitors [TKIs] plus immuno- oncology [IO], [such as] axitinib [Inlyta] plus pembrolizumab [Keytruda], cabozantinib [Cabometyx] plus nivolumab, or lenvatinib [Lenvima] plus pembrolizumab, those are approved across all IMDC risk groups, so when I’m seeing a patient who’s [presenting with] metastatic disease, among the fi rst things is I ask is, what is their risk score?” said Xu.
Kathryn Beckermann, MD, PhD, an oncologist at Vanderbilt University Medical Center in Nashville, Tennessee, drew the same conclusion from the topic of biomarkers in RCC to the utility of risk scores.
“If you ask a treating RCC expert what the applicable laboratory-based biomarker is to guide decision-making and treatment, the answer is we do not have one. But starting from there, I think we have lots of ongoing opportunities. We do have clinically validated risk stratification. The IMDC and the Memorial Sloan Kettering guides, among others, look at a mixture of laboratory and clinical factors, and enable clinicians to estimate patient biology and prognosis,” Beckermann said.
In addition to the IMDC and MSK risk scores, sarcomatoid or rhabdoid findings on histopathologic evaluation provide prognostic information.3
Xu considers these pathologic features as important as the risk models in terms of patient prognosis and treatment consideration.
“The second clinical risk stratifier, which I think is extremely important, is the presence or absence on pathology of sarcomatoid or rhabdoid features. This is something that a good pathologist will usually put into the report from a nephrectomy. And this is just as important as the IMDC risk score for first-line treatment. Because we’ve seen time and time again, across numerous phase 3 randomized trials, that patients with sarcomatoid features on their pathology, first of all do worse on average, so it’s prognostic in the sense that they survive a shorter time, but they do much better when they’re treated with immune checkpoint inhibitors,” said Xu.
Potential Biomarkers
What, in addition to risk models and sarcomatoid or rhabdoid pathology, can provide needed prognostic information for a patient?
“The next biomarker that I think we can still use as informational but has never become a required companion diagnostic test is PD-L1 [expression]. We know that, based on CheckMate 214 [NCT02231749] data, PD-L1 expression does suggest an enrichment of patients who will respond. It doesn’t mean that if they’re PD-L1 negative that they won’t—that’s the challenge. That can be due to biopsy bias, heterogeneity of kidney cancer, the antibody used, all those caveats that go into PD-L1 staining,” Beckermann told Targeted Therapies in Oncology™.6
Expanding on that diffi culty associated with immunohistochemical assessment of PD-L1 protein expression, Xu observed that “the signal is really inconsistent. PD-L1 [expression] has been associated with a higher chance of response in randomized trials. The association is weaker in other randomized trials, and patients who are negative for PD-L1 expression still seem to have significant benefi t from immune checkpoint inhibition. So, this is one of those biomarkers that probably has some signal, but the signal is very inconsistent and the signal is nowhere near strong enough to deny someone the benefit of PD-L1.” Next to IMDC, in terms of research and validation, “we have the most information on PD-L1 testing just because of the development of checkpoint inhibitors over the last 10 years. So that gets us to things that are really exciting now and going into the future,” Beckermann said.
Vanderbilt University Trial
Indeed, exciting research on potential biomarkers in the metastatic RCC space is ongoing. “I will say up front that I am biased because we just opened a biomarker design clinical trial,” Beckermann said.
“I think it’s an effort to start moving the field towards having a biomarker. Our trial is based on the IMmotion150 [NCT01984242] and IMmotion151 [NCT02420821] data that tested bevacizumab [Avastin] and atezolizumab [Tecentriq] and asked: Can we correlate biology and gene expression data with response to treatment? And we’re not the only ones—other studies have now gone on to look at similar gene expression patterns. In Europe, the BIONIKK trial [NCT02960906] was a prospective attempt at looking at gene expression and assigning treatment options based on gene expression signatures. So that was a great step in prospective validation and development of biomarkers,” said Beckermann.7-9
“We’re looking at if patients have an angiogenically driven tumor based on their gene expression vs having more of an immune-responsive biology. Those are things like T-effector function clusters that would be suggestive of responding to a checkpoint inhibitor," Beckerman said.
“We’re assigning ipilimumab plus nivolumab to patients who have these immune-based clusters, and we’re assigning cabozantinib plus nivolumab for patients who have an angiogenically driven gene expression pattern. We hope to learn more about the biology driving the remaining gene expression patterns and use this biology to identify novel therapeutic options,” Beckermann said.
Other Potential Biomarkers
Other emerging biomarkers in RCC that Xu finds interesting include studies involving the gut microbiome.
“Several groups have published several very provocative papers looking at the gut microbiome of patients with kidney cancer showing that certain bacteria are associated with higher chance of response to immunotherapy. And there was a recent phase 1 trial [NCT03829111] that was published this year showing that when they added a gut microbiome product, CBN588, to patients who received ipilimumab plus nivolumab, patients who got [CBN588] had a higher response rate with a hazard ratio of 0.15 for progression-free survival. That was very provocative and shows that maybe we can intervene at the gut microbiome and it could be a predictive biomarker that’s also an intervention,” said Xu.10-12 In NCT03829111, progression-free survival was signifi cantly longer in patients receiving nivolumab–ipilimumab with CBM588 than without (12.7 months vs 2.5 months, respectively; 95% CI, 0.05-0.47; P = .001) (see pages 36-37).
“There’s a lot of data on kidney cancer and PBRM1 [mutations], which [are] fairly common, and there were a couple of papers in Science 4 years ago as well as some other papers before [showing] that PBRM1 mutations seem to promote response to immunotherapy across trials. That’s another investigational biomarker that is still kind of in the works,” said Xu.13
There is also interest in fi nding peripheral blood biomarkers in RCC. However, Beckermann said, “kidney cancer doesn’t seem to have the same shedding rate that some of the other tumor types do, so that’s going to be a challenge. But as those methods are being developed, I’m hopeful that there might be a noninvasive, peripheral blood method of monitoring biology over time.” As a part of that effort, Xu and other researchers have begun exploring kidney injury molecule-1 (KIM1) as a potential plasma biomarker in the early RCC setting.14,15 Still others are studying circulating tumor cells with respect to the nuclear protein Ki-67 as a potential prognostic biomarker in RCC.16 The urgent need for predictive biomarkers in metastatic RCC may drive additional studies of biomarkers in combination therapeutic trials, which could help optimize personalized therapies for RCC.1
REFERENCES:
1. Sarkis J, Assaf J, Alkassis M. Biomarkers in renal cell carcinoma: towards a more selective immune checkpoint inhibition.Transl Oncol. 2021 Jun;14(6):101071. doi: 10.1016/j.tranon.2021.101071.
2. McGuire BB, Fitzpatrick JM. Biomarkers in renal cell carcinoma.Curr Opin Urol. 2009 Sep;19(5):441-6. doi: 10.1097/MOU.0b013e32832f0c68.
3. Tucker MD, Rini BI. Predicting response to immunotherapy in metastatic renal cell carcinoma. Cancers(Basel). 2020 Sep 18;12(9):2662. doi: 10.3390/cancers12092662.
4. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology Kidney Cancer. Version 3.2023. September 22, 2022. Accessed October 31, 2022. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
5. Noe A, de Bruijn RE, Blank C, Horenblas S, Haanen J, Bex A. Comparison of pre-treatment MSKCC and IMDC prognostic risk models in patients with synchronous metastatic renal cell carcinoma treated in the era of targeted therapy. World J Urol. 2016 Aug;34(8):1067-72. doi: 10.1007/s00345-016-1769-7.
6. Okita K, Hatakeyama S, Tanaka T, et al. Impact of disagreement between two risk group models on prognosis in patients with metastatic renal-cell carcinoma. Clin Genitourin Cancer. 2019 Jun;17(3):e440-e446. doi: 10.1016/j.clgc.2019.01.006.
7. Motzer RJ,Tannir NM, McDermott DF, et al; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma.N Engl J Med. 2018 Apr 5;378(14):1277-1290. doi: 10.1056/NEJMoa1712126.
8. Epaillard N, Simonaggio A, Elaidi R, et al. BIONIKK: A phase 2 biomarker driven trial with nivolumab and ipilimumab or VEGFR tyrosine kinase inhibitor (TKI) in naïve metastatic kidney cancer. Bull Cancer. 2020 Jun;107(5S):eS22-eS27. doi: 10.1016/S0007-4551(20)30283-6.
9. Powles T, Atkins MB, Escudier B,et al. Efficacy and safety of atezolizumab plus bevacizumab following disease progression on atezolizumab or sunitinib monotherapy in patients with metastatic renal cell carcinoma in IMmotion150: a randomized phase 2 clinical trial. Eur Urol. 2021 May;79(5):665-673. doi: 10.1016/j.eururo.2021.01.003.
10. Rini BI, Powles T, Atkins MB, et al; IMmotion151 Study Group. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre,open-label, phase 3, randomised controlled trial.Lancet. 2019 Jun 15;393(10189):2404-2415. doi: 10.1016/S0140-6736(19)30723-8.
11. Derosa L, Routy B, Fidelle M, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients.Eur Urol. 2020 Aug;78(2):195-206. doi: 10.1016/j.eururo.2020.04.044.
12. Dizman N, Hsu J, Bergerot PG, et al. Randomized trial assessing impact of probiotic supplementation on gut microbiome and clinical outcome from targeted therapy in metastatic renal cell carcinoma.Cancer Med. 2021 Jan;10(1):79-86. doi: 10.1002/cam4.3569.
13. Dizman N, Meza L, BergerotP, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial.Nat Med. 2022 Apr;28(4):704-712. doi: 10.1038/s41591-022-01694-6.
14. Liu XD, Kong W, Peterson CB, et al. PBRM1loss defines a nonimmunogenic tumor phenotype associated with checkpoint inhibitor resistance in renal carcinoma.Nat Commun. 2020 May 1;11(1):2135. doi: 10.1038/s41467-020-15959-6.
15. Scelo G, Muller DC, Riboli E, et al. KIM-1 as a blood-based marker for early detection of kidney cancer: a prospective nested case-control study.Clin Cancer Res. 2018 Nov 15;24(22):5594- 5601. doi: 10.1158/1078-0432.CCR-18-1496.
16. Xu W, Puligandla M, Halbert B, et al. Plasma KIM-1 Is associated with recurrence risk after nephrectomy for localized renal cell carcinoma: a trial of the ECOG-ACRIN Research Group (E2805).Clin Cancer Res. 2021 Jun 15;27(12):3397-3403. doi: 10.1158/1078-0432.CCR-21-0025.
17. Song J, Yu Z, Dong B, et al. Clinical significance of circulating tumour cells and Ki-67 in renal cell carcinoma.World J Surg Oncol. 2021 May 25;19(1):156. doi: 10.1186/s12957-021-02268-5
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen