Antibiotics Reduce ICI Efficacy for Patients With Urological Cancers
Patients with metastatic renal and urothelial bladder cancer who receive antibiotics concomitantly with immune checkpoint inhibitors have shorter progression-free survival and overall survival rates than patients who do not, according to a poster presented at the European Congress on Immunotherapies in Cancer™ conference, hosted by Physicians’ Education Resource®, LLC, September 21 and 22, 2018, in Barcelona, Spain.
Patients with metastatic renal and urothelial bladder cancer who receive antibiotics concomitantly with immune checkpoint inhibitors (ICIs) have shorter progression-free survival (PFS) and overall survival (OS) rates than patients who do not, according to a poster presented at the European Congress on Immunotherapies in Cancer™ conference, hosted by Physicians’ Education Resource®, LLC, September 21 and 22, 2018, in Barcelona, Spain.
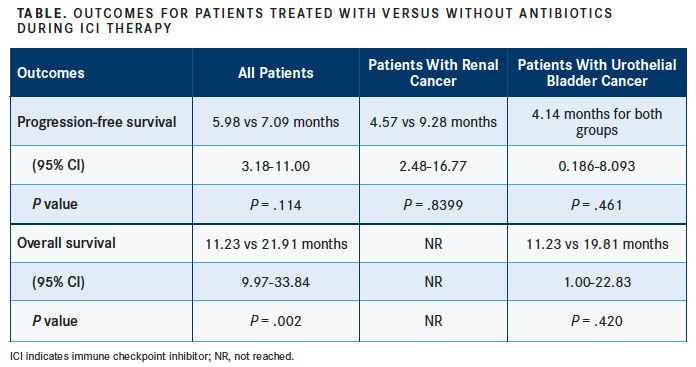
The retrospective analysis, conducted by investigators at the Vall d’Hebron Institute of Oncology, found that the use of antibiotics was associated with a 10.68-month reduction in OS compared with patients who did not receive antibiotics while undergoing treatment with antiPD-1 or anti–PD-L1 therapy (TABLE).
“There are no clear biomarkers for the effectiveness of ICIs. One of the most common is PD-L1 expression that has been used as a prognostic factor and predictor of ICI response. It is not, however, a standardized marker. This is understandable since the immune system can be modulated by different tumors as well as host-related elements,” said the study’s principal investigator, Joan Carles, MD, PhD, of the Vall d’Hebron Institute of Oncology’s Genitourinary, CNS Tumors, Sarcoma & Cancer of Unknown Primary Site Group, in an interview with Targeted Therapies in Oncology.
Carles went on to describe how antibiotics disrupt gut microbiota diversity which, in turn, affects antitumor responses. “The gut microbiota is made up of a wide variety of bacteria, viruses, fungi, and other single-cell [organisms] that…can modulate different processes within the body, including the immune system,” he said.
This disruption may alter the effectiveness of ICIs in treating cancer. “If we treat a patient with antibiotics, we will trigger alterations in the microbiota by killing bacteria. This can result in immunoactivity and facilitate ICI responses,” he said.
Investigators gathered data for 80 patients with metastatic renal (n = 28) or urothelial bladder (n = 52) cancer who were treated with PD-1 or PD-L1 inhibitors as monotherapy or in combination with other agents from November 2014 to February 2018. Patients who received antibiotics up to 1 month before the first injection of ICI, during their treatment, or up to 2 months after their last injection were compared with those patients who did not.
The median patient age at diagnosis was 67 years and a majority of patients (75%) were male. Fifty-two patients (65%) were being treated with antiPD-1 and anti–PD-L1 monotherapy, with the remainder of patients receiving combination therapy, including CTLA-4 (n = 7) and antiangiogenesis therapy (n = 16).
For all patients, the median PFS for those treated with antibiotics was 5.98 months, compared with 7.09 months in patients not treated with antibiotics (95% CI, 3.18- 11.00; P = .114).
Antibiotic use during ICI therapy was associated with worse OS rates, compared with patients who did not receive them (95% CI, 9.97-33.84; P = .002). The median OS in patients treated with antibiotics was 11.23 months, compared with 21.91 months with no antibiotics.
Differences in PFS were seen when patients were broken into subgroups based on their disease type. In patients with renal cancer, median PFS for antibiotic use was 4.57 months, compared with 9.28 months without antibiotic treatment (95% CI, 2.48-16.77; P = .8399).
For patients with urothelial bladder cancer, the observed median PFS in both groups was 4.14 months (95% CI, 0.186-8.093; P = .461). But patients who did not receive antibiotics had better rates of OS than those who had (19.81 vs 11.23 months; 95% CI, 1.00-22.83; P = .420).
The objective response rate (ORR) in all patients with renal cancer was 53.6%, with all patients experiencing a partial response (PR). In patients with urothelial bladder cancer, the ORR was 48.1%, with 17.3% experiencing a complete response and 30.8% having a PR.
A total of 45 patients received antibiotic treatment, and the most commonly prescribed medications were β-lactams and quinolones. Patients were most likely to receive antibiotic therapy for the treatment of respiratory and urinary tract infections, and 13.8% of patients received prophylactic antibiotics.
Asked about future plans for clinical trials or studies to further assess the impact of antibiotics on patients receiving ICIs, Carles said “different studies are planned to assess microbiota and its effect on the immune system and correlation with ICI treatment.
Reference:
González M, Morales-Barrera R, Suarez C, et al. Clinical impact of the use of antibiotics in patients with metastatic renal and urothelial bladder cancer treated with immune checkpoint inhibitors. Poster presented at: European Congress on Immunotherapies in Cancer™, Physicians’ Education Resource®, LLC; September 21-22, 2018; Barcelona, Spain.
Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More