2020 ASCO Recap: Experts Review Most Impactful Oncology/Hematology Data
Following the 2020 ASCO Virtual Scientific Program, Targeted Oncology spoke with oncologists from various fields to hear their insights on what data they found most impactful in this year’s meeting.
For the American Society of Clinical Oncology (ASCO) Annual Meeting, oncologists, physicians, nurses, and other professionals flock to the windy city of Chicago, IL, annually from around the world for the presentation of new data across cancer types. However, as the coronavirus disease 2019 (COVID-19) pandemic swept the nation, ASCO converted the meeting to a virtual format for the ASCO Virtual Scientific Program, demonstrating the organization’s commitment to accelerating progress for millions of patients with cancer around the world.
Although in-person communication and collaboration with oncology minds from around the world is a big part of the meeting, this year’s 3-day virtual scientific meeting reached more than 42,000 attendees from over 130 countries to date. Attendees were able to view 5300 abstracts online throughout the weekend, which included more than 100 on-demand and broadcast sessions and over 2300 presentations. According to an announcement from ASCO, the content from the scientific program has been viewed more than 2.5 million times to date.1
Petros Grivas, MD, PhD

“It is a very different experience compared with what we are used to. I think everyone looks forward to ASCO mainly for networking. It is different to be there [in person] to talk about abstracts, ask questions, and have this face-to-face interaction with colleagues and academics, as well as community oncologists and industry,” Petros Grivas, MD, PhD, physician, Seattle Cancer Care Alliance; associate professor, Department of Medicine, Division of Oncology and clinical director, Genitourinary Cancers Program, University of Washington School of Medicine; and associate member, Clinical Research Division, Fred Hutchinson Cancer Center, told Targeted Oncology. “We missed that this year, but I think ASCO did the right thing. We are pleased to at least have ASCO virtually. It is important that we are able to experience it virtually this year. Maybe it’s suboptimal, but it’s necessary during this pandemic.”
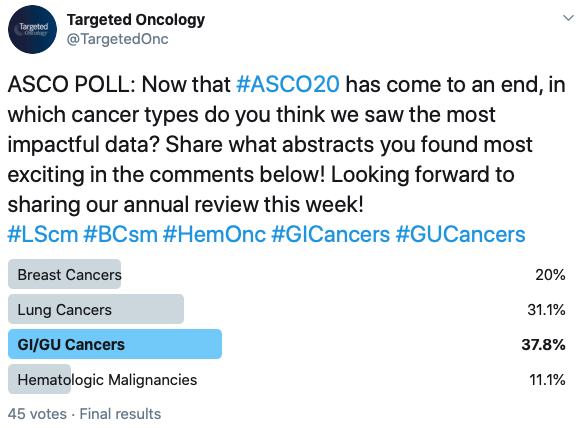
Following the 2020 ASCO Virtual Scientific Program, Targeted Oncology spoke with many oncologists to hear their insights on what data they found most impactful in this year’s meeting. On Twitter, Targeted Oncology followers shared their thoughts on a poll, which showed most voters found the data in genitourinary (GU) and gastrointestinal (GI) cancers most impactful, but there was a great amount of interest in lung cancers, breast cancers, and hematologic malignancies, as well as gynecologic oncology.
“This meeting was particularly groundbreaking in a way that connected many oncologists with industry, surgical departments, and others throughout the world virtually. Overall, I think it was a great success, and kudos to ASCO for organizing such a diverse and exciting meeting,” Yelena Y. Janjigian, MD, a medical oncologist at Memorial Sloan Kettering Cancer Center, told Targeted Oncology. “In some ways, it was bittersweet because we are so used to seeing each other, connecting and cross-pollinating ideas, but overall considering the current climate, it was a great success.”
Although there were many impactful data presented in this year’s virtual program, the phase 3 JAVELIN Bladder 100 study was particularly exciting for many oncologists at this year’s meeting, including those outside of the GU cancer space.
Yelena Y. Janjigian, MD

“This was an abstract that all GI oncologists should consider,” Janjigian said. “Notably, the trials in GU cancers of maintenance therapy with immunotherapy have not led to success, but given the trial design, sample size, and the overall patient selection in bladder cancer that were very specifically conducted, [this trial] gives us pause and makes us wonder whether or not we should go back to the drawing board and rethink the strategy of anti–PD-1/PD-L1 maintenance therapy in our gastric cancers.”
GU Cancers
Frontline Avelumab Maintenance Prolongs Survival in Urothelial Carcinoma
Avelumab (Bavencio) combined with best supportive care extended overall survival (OS) by more than 7 months, leading to a median OS of 21.4 months, in patients with locally advanced or metastatic urothelial carcinoma compared with a median OS of 14.3 months with best supportive care alone, according to data from the phase 3 JAVELIN Bladder 100 study (NCT02603432). The agent also reduced the risk of death by 31%.
Neeraj Agarwal, MD

“Frontline maintenance therapy with avelumab after completion of platinum therapy significantly and dramatically improves OS as well as progression-free survival (PFS) versus best supportive care in patients with advanced bladder (urothelial) cancer,” said Neeraj Agarwal, MD, professor, Huntsman Cancer Center, University of Utah. “This is a new standard of care.”
In April 2020, the FDA granted a Breakthrough Therapy designation to avelumab and accepted a supplemental Biological License Application for the drug based on the data from this study. The application seeks approval of the PD-L1 inhibitor as treatment of patients with locally advanced or metastatic urothelial cancer in the frontline setting.
Toni K Choueiri, MD

“[This is the] first time seeing a switch maintenance trial with immunotherapy in solid tumors showing a strong OS benefit,” Toni K Choueiri, MD, director, Lank Center for Genitourinary Oncology and the Kidney Cancer Center, Dana-Farber Cancer Institute, told Targeted Oncology. “Avelumab can become a standard after chemotherapy (platinum-based doublets) induction for patients that do not show a clear progressive disease.”
Choueiri noted that some questions still exist surrounding this regimen. “Some open questions [include] do we need to treat with a PD-L1 inhibitor until progression, or [is a] shorter duration possible? Should we intensify therapy in future trials in patients with visceral metastases? Who are the patients that we can pick and observe and give systemic therapy upon progression?”
Clinically Meaningful Activity Observed With Combination Cabozantinib and Atezolizumab in mCRPC
Cabozantinib (Cabometyx) plus atezolizumab (Tecentriq) induced clinically meaningful activity in patients with metastatic castration-resistant prostate cancer (CRPC), including patients with high-risk clinical features in the multicenter phase 1b COSMIC-021 study (NCT03170960).
The objective response rate (ORR) was 32% among patients with metastatic CRPC, but the ORR was 33% among those with high-risk features, which included those with visceral metastases and/or extra-pelvic lymph node metastases.
“The combination of cabozantinib and atezolizumab demonstrated a tolerable safety profile and clinically meaningful activity in men with metastatic CRPC progressing on a novel hormonal therapy (enzalutamide or abiraterone or both) who have very limited options,” said Agarwal. “ORR was 33% per RECIST v1.1 with a median duration of response of 8.3 months. An additional 47% of patients had stable disease per RECIST v1.1. So, the overall disease control rate—ORR plus stable disease rate—was 80% in this study with the combination cabozantinib and atezolizumab.”
Relugolix (Relumina) treatment is superior over leuprolide (Lupron) in patients with advanced prostate cancer, according to the phase 3 HERO (NCT03085095) study findings. Testosterone suppression was sustained through 48 weeks with relugolix; there was also fast testosterone recovery after discontinuation and a 50% reduction in major adverse cardiovascular events.
The agent also achieved castration as early as day 4 in this study, which suggests this agent may have potential as a new standard for testosterone suppression in patients with advanced disease.
Agarwal commented on this study, saying it is an appealing study with, “remarkably lower major adverse cardiovascular events with relugolix, despite more effective castration. Relugolix deserves discussion with patients with advanced prostate cancer on single-agent ADT and especially in those with significant cardiovascular illnesses.”
DFS Improvement Falls Short With Anti–PD-L1 in Muscle-Invasive Urothelial Cancer
Adjuvant treatment with anti–PD-L1 therapy atezolizumab demonstrated a numerically increased but not statistically significant improvement in disease-free survival (DFS) in patients with muscle-invasive urothelial carcinoma compared with observation in the phase 3 IMvigor010 study (NCT02450331).
“This is a negative trial even in important subgroups,” Choueiri noted. “[There was] no benefit in PD-L1–positive patients or even higher-risk (pN+) patients, [so we] cannot recommend adjuvant therapy with immunotherapy outside a trial. Please support the AMBASSADOR ALLIANCE trial and PROOF302 (FGFR inhibitor in a selected group). Both are ongoing and accruing trials in similar populations of high-risk muscle-invasive urothelial carcinoma.”
This is the first phase 3 study of an immune checkpoint inhibitor in this patient population. Evaluations of the efficacy of atezolizumab remain ongoing in this setting. Other clinical trials are looking at the agent as a monotherapy or in combination with other treatments for a variety of disease settings in urothelial carcinoma.
Survival Benefits Are Maintained With Frontline Pembrolizumab/Axitinib in Advanced RCC
The combination of pembrolizumab (Keytruda) plus axitinib (Inlyta) continued to show clinically meaningful improvements in both PFS and OS as treatment of patients with treatment-naïve advanced renal cell carcinoma (RCC). The median OS had not been reached at a minimum of 23 months of follow-up with the combination compared with 35.7 months with sunitinib (Sutent). The 1- and 2-year OS rates with the combination were 90% and 74% compared with 79% and 66% with sunitinib, respectively.
“Pembrolizumab/axitinib is an approved regimen in front-line advanced RCC. This 23-month update shows that the OS remains maintained overall with higher rates of complete response (CR). The HR of OS in the update [is] 0.68 from 0.53 originally at 7-month follow-up,” said Choueiri. “At the write-up of this piece, that combination is the only FDA-approved VEGF plus immunotherapy combination with an OS benefit.”
Salvage Nivolumab/Ipilimumab Appears Promising in Clear Cell Renal Cell Carcinoma
According to results from the phase 2 HCRN-GU16-260 study, salvage nivolumab (Opdivo) plus ipilimumab (Yervoy) is not only a contender for frontline treatment of patients with clear cell RCC, but it may be more beneficial than nivolumab monotherapy. This is particularly true for patients with intermediate and poor-risk disease, as well as those with sarcomatoid kidney cancer, in which the salvage combination induced a higher ORR, longer PFS, longer duration of response, and more CRs.
“This work, as well as others presented during the same ASCO meeting and at 2019 ESMO, show that a response-adapted strategy, starting by nivolumab and adding ipilimumab later, may not be ideal [with] very low rates, if any, of CRs; attrition rate high; and ORR lower than nivolumab plus ipilimumab as a front-line combination in an immunotherapy-naïve population,” Choueiri said.
Other Impactful Data in GU Cancers
“Three abstracts, SPARTAN/PROSPER/ARAMIS in M0 [nonmetastatic] CRPC, all show OS benefit (gold standard) with enzalutamide/apalutamide/darolutamide,” said Agarwal. He also noted that these are, “compelling data for offering these agents to all eligible pts with nonmetastatic CRPC. [We] need comparative effectiveness including QOL and cost.”
In the phase 3 ARAMIS study (NCT02200614), patients with nonmetastatic CRPC receiving darolutamide (Nubeqa) plus androgen deprivation therapy (ADT) had a 31% reduction for the risk of death compared with placebo and ADT. After a median of 29 months of follow-up, the 3-year OS rate was 83% with the darolutamide regimen compared with 77% with placebo (HR, 0.69; 95% CI, 0.53-0.88; P = .003).
According to data from the phase 3 SPARTAN study (NCT01946204), apalutamide (Erleada) in combination with ADT also led to a significant improvement in OS compared with ADT alone and placebo in patients with nonmetastatic CRPC. The median OS with the combination regimen was 73.9 months versus 59.9 months for ADT plus placebo (HR, 0.784; P = .0161). Apalutamide also significantly increased time to cytotoxic chemotherapy (HR, 0.629; P = .0002) when compared with the placebo arm.
GI Cancers
Pembrolizumab Extends PFS in Newly Diagnosed MSI-H/dMMR mCRC
Pembrolizumab doubled the PFS in patients with newly diagnosed microsatellite instability-high (MSI-H)/mismatch repair deficient metastatic colorectal cancer (CRC) compared with chemotherapy in the phase 3 KEYNOTE-177 trial (NCT02563002).
“The most important data were the findings from the first-line MSI-H CRC study, KEYNOTE-177, where investigators from [an] international collaboration compared chemotherapy in the first-line setting to pembrolizumab,” Janjigian said. “This was an exciting and groundbreaking abstract showing a dramatic disease-free survival benefit for use in the first-line setting.”
The median PFS was 16.5 months with pembrolizumab (95% CI, 5.4-32.4) compared with 8.2 months with standard chemotherapy with or without bevacizumab (Avastin) or cetuximab (Erbitux), which led to a 40% reduction in the risk of disease progression or death (HR, 0.60; 95% CI, 0.45-0.80; P = .0002).
“This is truly a practice-changing abstract, highlighting the importance of biomarker analysis in CRC. In my opinion, this will change practice,” Janjigian said.
HER2+ Colorectal and Gastric/GEJ Cancers Demonstrate Responses to Fam-Trastuzumab Deruxtecan Therapy
Fam-trastuzumab deruxtecan-nxki (Enhertu) induced responses in patients with HER2-positive metastatic CRC and advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma in 2 phase 2 studies, DESTINY-CRC01 (NCT03384940) and DESTINY-Gastric01 (NCT003329690).
“This was a big year for DESTINY trials,” Janjigian said. “Trastuzumab deruxtecan, the new [HER2] inhibitor on the market in GI cancers, is a drug that is already FDA-approved in breast cancer and is now being developed in both CRC and HER2-positive gastric cancer. It showed exciting activity, a high response rate, and durable PFS in both settings.”
In the DESTINY-CRC01 trial, the ORR was 45.3% in patients with HER2-positive metastatic CRC who were refractory to standard treatment (95% CI, 31.6%-59.6%), and the median duration of response had not yet been reached. In the DESTINY-Gastric01 trial, the ORR was 51.3% with this agent compared with 14.3% with chemotherapy.
“Compared with chemotherapy in the third-line setting, trastuzumab deruxtecan had dramatic improvements in survival and response rate. Based on these data, the FDA granted Priority Review to this agent in the third-line setting.”
Pembrolizumab Noninferior to Chemotherapy in Advanced Gastric Cancer in KEYNOTE-062
In the phase 3 KEYNOTE-062 study, pembrolizumab demonstrated similar benefit in terms of OS with pembrolizumab versus chemotherapy in the first-line setting of patients with gastric of GEJ adenocarcinoma. The checkpoint inhibitor induced clinically meaningful efficacy in patients with (CPS) ≥10, as well as more durable responses compared with chemotherapy.2
“The data from KEYNOTE-062, which looked at pembrolizumab or pembrolizumab with chemotherapy, showed that in the MSI-high cohort, the response rates are higher with chemotherapy plus pembrolizumab, so perhaps in that population of patients who are hyper progressors, the addition of pembrolizumab is warranted,” Janjigian said. “The other alternative approach that has been discussed is the combination of anti–PD-1 and anti–CTLA-4 blockade as data has been published in CRC that nivolumab plus ipilimumab has a higher response rate in combination in MSI-H tumors. Nonetheless, this was a big splash at ASCO, highlighting the importance of biomarker testing and knowing the MSI status in your patients, both in CRC and other disease types.”
Preliminary Data Show Novel RGX-202 Is Tolerable in Advanced Gastrointestinal Solid Tumors
As monotherapy, RGX-202 was well tolerated and showed efficacy signals in higher-dose cohorts in patients with KRAS-mutant CRC; in combination with FOLFIRI, no dose-limiting toxicities were observed to date, although the last cohort remains ongoing in the phase 1 study (NCT03597581). With the combination regimen, investigators noted a prolonged disease control in 80% of the patients who were evaluable.3
“The KRAS story fizzled at ASCO, particularly in CRC,” Janjigian said. “Interestingly, RGX-202 is a novel first-in-class inhibitor of the SLC6a8/CKB pathway, which is important in its cancer metabolism. In this preliminary phase 1 study, we saw clinical activity in a variety of solid tumors, including KRAS-mutant CRC. This study is now currently being expanded to combination chemotherapy with FOLFIRI plus RGX202. This is a new drug that we should maybe look out for.”
The phase 1b/2 biomarker-directed expansion of the study is planned to evaluate this agent in patients with advanced CRC, and patients will be selected based on CKB biomarker positivity.
Lung Cancers
Antonio Passaro, MD, PhD

Adjuvant Osimertinib Leads to Significant Boost in DFS in Early-Stage EGFR+ NSCLC
In the randomized phase 3 ADAURA study, osimertinib (Tagrisso) led to an 83% reduction in the risk of disease recurrence or death in patients with stage II to IIIA EGFR-positive non–small cell lung cancer (NSCLC). Among patients with stage IB to IIIA disease, the risk of disease recurrence or death was reduced by 79% (HR, .021; 95% CI, 0.16-0.28; P <.0001).
Osimertinib was well tolerated in the study and demonstrated a consistent safety profile. The most frequent adverse events (AEs) included diarrhea, paronychia, and dry skin, but these were all mostly grades 1 or 2.
“The results showed that adjuvant osimertinib significantly improved disease-free survival (DFS) compared with placebo, in patients with stage IB to IIIA NSCLC harboring EGFR mutations after radical surgery and chemotherapy, if indicated. The HR for DFS (primary endpoint) in patients with stage II to IIIa was 0.17. Although these results are extremely positive in favor of osimertinib, many questions came out,” Antonio Passaro, MD, PhD, a medical oncologist and thoracic oncologist at the European Institute of Oncology, Milan, Italy, told Targeted Oncology.
“There is no consensus among all the stockholders discussing DFS and the missing value of OS, considering also the limitations of [the] control arm at disease progression, optimal treatment duration, and related drug cost,” Passaro added. “These data do not clarify if adjuvant osimertinib is able to cure our patients, but [we are] sure there is a significantly delay in the disease recurrence, regardless of clinical stages with a major achievement in late stage, as stage III (HR of 0.12).”
Joshua Bauml, MD

“While these data are very exciting, we must be cautious. The OS data are immature, and nearly half of the enrolled patients did not receive adjuvant chemotherapy, which would be the standard of care in node-positive disease,” Joshua Bauml, MD, assistant professor of medicine, Division of Hematology/Oncology, Perelman School of Medicine at the University of Pennsylvania, told Targeted Oncology. “I look forward to seeing the publication of these data as we discuss this important potential option for our patients.”
Trastuzumab Deruxtecan Demonstrates Favorable Clinical Activity in HER2+ NSCLC
Fam-trastuzumab deruxtecan-nxki (Enhertu) demonstrated favorable clinical activity with high objective response rates (ORRs) and durable responses as treatment of patients with HER2-positive NSCLC in the phase 2 DESTINY-Lung01 study.
“HER2 exon 20 mutations are a recurrent driver alteration that affects patients with NSCLC at a frequency similar to ALK and MET, which have already led to FDA-approved targeted therapies,” Bauml said. “To date, however, available HER2-targeted treatments have led to largely disappointing results in this patient population. Last year, we saw some efficacy from ado-trastuzumab emtansine (T-DM1; Kadycla), but responses were brief. This year, we saw another antibody-drug conjugate with substantial efficacy; trastuzumab deruxtecan led to a very impressive ORR.”
The ORR by independent central review was 61.9% (95% CI, 45.6%-76.4%), and the median duration of response had not yet been reached (95% CI, 5.3 months-not evaluable [NE]). The estimated PFS was 14.0 months. The disease control rate (DCR) was 90.5% (95% CI, 77.4%-97.3%).
“The presentation discussing the results of the ongoing DESTINY-Lung01 trial on trastuzumab deruxtecan (6.4 mg/kg) in patients with HER2-mutant NSCLC, in the pretreated setting, was the most important presentation looking at the future,” Passaro said. “Unfortunately, safety profile appeared the Achilles' heel of this very active drug, showing 23.8% of discontinuation due to treatment-related adverse events and 38.1% of dose reduction [being] drug-related, but the subsequent development of the drug is moving on to a lower dosage (5.4 mg/kg), that I hope can confirm the efficacy with a better safety profile.”
Based on this study, the FDA granted a Breakthrough Therapy designation to trastuzumab deruxtecan for the treatment of patients with metastatic NSCLC who are HER2-mutated with disease progression on or after platinum-based therapy.
“To date, these results validate HER2 mutations as druggable targets in lung cancer and confirm the high potential of trastuzumab deruxtecan in this setting, accounting for approximately 2% to 4% of patients with NSCLC,” Passaro concluded on the DESTINY-Lung01 trial.
“Even more important, these responses seemed to be quite durable,” Bauml said. “I look forward to more data from this agent.”
Durable Responses Elicited with Nivolumab Plus Ipilimumab in Advanced NSCLC
Frontline treatment with nivolumab (Opdivo) plus ipilimumab (Yervoy) demonstrated durable and long-term efficacy benefits in patients with advanced NSCLC regardless of PD-L1 expression in the CheckMate 227 study. The median OS was 17.1 months with the combination compared with 15.7 months with nivolumab alone and 14.9 months with chemotherapy alone. The 3-year OS rates were 33% with nivolumab plus ipilimumab, 29% with nivolumab alone, and 22% with chemotherapy. This combination has been approved by the FDA for the treatment of patients with metastatic or recurrent NSCLC whose tumors express PD-L1 (≥1%), as determined by an FDA-approved test, and who do not have an EGFR or ALK tumor aberration.
“Based on the data from CheckMate 227, the combination of nivolumab and ipilimumab is approved in patients with PD-L1–positive disease. Based on the CheckMate 9LA trial, the combination of nivolumab/ipilimumab and 2 cycles of platinum doublet chemotherapy was approved regardless of the PD-L1 status,” Bauml said. “In looking at these curves it is clear that these are active regimens with a toxicity profile that is comparable to available regimens.”
OS Benefit Observed With Frontline Nivolumab/Ipilimumab Plus Limited Chemotherapy in NSCLC
Frontline nivolumab plus ipilimumab with 2 cycles of a platinum doublet chemotherapy demonstrated superior OS versus chemotherapy alone in patients with metastatic or recurrent NSCLC, regardless of PD-L1 expression or tumor histology (HR, 0.69; 96.71% CI, 0.55-0.87; P = .0006), according to the findings from the CheckMate 9LA clinical trial.
“The CheckMate 9LA was 1 of the most waited presentations, after the press release. The trial confirmed a statistically significant improvement of all the efficacy endpoints, including OS for the combo of nivolumab plus ipilimumab given concomitantly with 2 cycles of chemotherapy for the first-line treatment of NSCLC, regardless [of] PD-L1 expression. Subgroup analysis showed that nearly every group benefited from the combo except never smokers and patients with ≥70 years.”
The FDA granted approval to this regimen on May 26, 2020, for the treatment of patients with metastatic or recurrent NSCLC who do have an EGFR or ALK genomic tumor aberration, regardless of histology or PD-L1 expression.
“Although these results are certainly positive, confirming the high efficacy of the experimental combination already approved by FDA, it seems difficult today to evaluate the real potential of this treatment, consider that as for CheckMate- 227, these trials did not use chemotherapy plus immunotherapy as control arm, which nowadays remains the standard of care,” Passaro said.
“The key question now is how we choose which patients should get ipilimumab, which should get chemotherapy, and which can just get PD-1/PD-L1 blockade alone,” Bauml concluded. “I am hopeful that future biomarker studies will allow us to personalize our use of immunotherapy for patients with lung cancer.”
Other Impactful Data in Lung Cancer
“Three abstracts discussed the role of TKIs (tyrosine kinase inhibitors) for [EGFR] exon 20–positive NSCLC, confirming a great interest for this orphan biological cluster, in which no targeted therapies are approved,” said Passaro. “Reported data from cohort 1 of the ZENITH-20 phase 2 study, evaluating poziotinib 16 mg [daily], confirmed as noted its activity in this setting, showing also clinical activity in central nervous system (CNS) metastases.”
Investigators noted that having received multiple prior lines of therapy did not impair responses with poziotinib, according to the findings from cohort 1 in the phase 2 ZENITH20 study (NCT03318939).
“Unfortunately, we know that this agent reported a significant rate of adverse events, to be considered as a limiting factor,” Passaro added.
“Amivantamab, an anti–EGFR-MET bispecific antibody, that already received FDA Breakthrough Therapy Designation in the pretreated setting, showed a durable response with a median duration of response of 10 months, an ORR of 36% and a median PFS of 8.3 months among all patients enrolled, demonstrating also a manageable safety profile,” Passaro said. “Waiting for a highly effective and specific exon 20 inhibitor, these results are very encouraging and I'm very intrigued to look forward [to] more robust data from this agent.”
“On the other hand, waiting for a specific target agent against exon 20 insertions, the results of the ECOG-ACRIN-5162 phase 2 trial showed that using osimertinib at a double dose (160 mg) achieved a mPFS of 9.6 months with a DCS and ORR of 82% and 24%, respectively,” said Passaro. “These very interesting results, based on 21 patients, need further evaluation, and clinical trials are planned to deeply investigate the role of osimertinib in this setting.”
In the phase 2 ECOG-ACRIN 5162 trial, a daily dose of osimertinib at 160 mg was well tolerated with clinical activity in patients with EGFR exon 20–mutant NSCLC.
“Waiting for TKI as a real standard of care in NSCLC carrying exon 20 insertions, testing all of our patients, and detecting these rare mutations should be recommended in daily clinical practice,” Passaro said.
In the RET fusion–positive treatment landscape, 2 clinical trials stood out to Passaro at this year’s meeting.
“Selpercatinib (LOXO-292) and pralsetinib (BLU-667) are 2 of the most significant advancements of recent years in the field of precision medicine, in particular as targeted agents in RET fusion-positive NSCLC. Data on CNS activity of selpercatinib, recently approved by FDA, were reported by Viviek Subbiah, MD, showing selpercatinib had marked intracranial antitumor activity in [patients with] RET fusion–positive NSCLC with CNS metastases,” Passaro said.
In the phase 1/2 LIBRETTO-001 study, selpercatinib demonstrated durable intracranial and antitumor activity in patients with RET fusion–positive NSCLC, and the agent was overall well tolerated.
“In the other abstract by Justin Gainor, MD, a registrational data set from the phase 1/2 ARROW [study] of pralsetinib were reported, showing an impressive 96% of patients with tumor reduction and an ORR of 65%,” said Passaro. “Results also confirmed a robust intracranial activity for these target agents.”
The phase 1/2 ARROW trial demonstrated significant efficacy with pralsetinib across various advanced solid tumors harboring RET alterations, which included patients with difficult-to-treat cancers.
“For our patients, being able to benefit from your extremely effective drugs such as pralsetinib and selpercatinib is a great step forward in the cure, in a very rare setting,” Passaro said. “All of these results presented this year, confirmed that at least 8 oncogenes should be tested for every new diagnosis of advanced NSCLC, especially for those with non-squamous histology. A broad-based NGS (next-generation sequencing) analysis should be a guarantee to all of our patients, and a common effort must be made to implement the diffusion of these methods in order to do not limit some treatments to a small patient population.”
Gynecologic Cancers
David O'Malley, MD

Maintenance Olaparib Provides Significant Long-Term OS Benefit in Relapsed Ovarian Cancer
Maintenance olaparib (Lynparza) led to an improved median OS of 12.9 months compared with placebo as treatment of patients with platinum-sensitive BRCA-mutant relapsed ovarian cancer in the final OS results of the phase 3 SOLO2/ENGOT-ov21 study. The median OS was 51.7 months with olaparib versus 38.8 months with placebo (HR, 0.74; 95% CI, 0.54-1.00; P = .0537). The PARP inhibitor also induced a PFS benefit of 13.6 months, with a median PFS of 19.1 months with olaparib versus 5.5 months with placebo (HR, 0.30; 95% CI, 0.22-0.41; P <.0001).
“The SOLO2 OS data were quite impressive and show that the significant improvement in PFS translated to a marked improvement in OS, [leading] to further evidence that patients with BRCA mutations need to have PARP inhibitors earlier on in the treatment course,” David O'Malley, MD, professor in the Department of Obstetrics and Gynecology at The Ohio State University College of Medicine, and director of the Division of Gynecologic Oncology at The Ohio State University Comprehensive Cancer Center–The James, told Targeted Oncology.
Mirvetuximab Soravtansine Shows Promise in Platinum-Agnostic Ovarian Cancer
The combination of mirvetuximab soravtansine plus bevacizumab (Avastin) demonstrated encouraging responses and a favorable safety profile as treatment of patients with platinum-agnostic ovarian cancer regardless of the platinum status in a study presented by Luck Gilbert, MD, MSc.
The combination led to an ORR of 47% in the entire cohort, but the ORR among those with high folate receptor alpha-expressing tumors was 64%. The ORR in the platinum-resistant subset was 59% and was 69% in those with platinum-sensitive disease.
“Mirvetuximab soravtansine continues to show marked response rates, particularly in those high folate receptor alpha expressors,” said O’Malley. “The agent, when combined with bevacizumab, is clearly beneficial to patients with ovarian cancer.”
The combination had a manageable and consistent safety profile with the known toxicity profiles of each agent.
Responses to Olaparib/Cediranib Combination in Ovarian Cancer Warrant Further Investigation
Cediranib in combination with olaparib and standard-of-care platinum-based chemotherapy demonstrated comparable activity with either olaparib or chemotherapy alone in the phase 3 NRG-GY004 study of patients with recurrent platinum-sensitive ovarian cancer. However, the trial missed its primary end point of PFS improvement.
“The trial presented on cediranib and olaparib confirms the combination is active in platinum-sensitive disease,” O’Malley said. “However, the trial did not meet its primary end point, thus it was not considered superior to a platinum-doublet.”
The median PFS for the combination was 10.4 months (HR, 0.856; 95% CI, 0.66-1.11; P = 0.08, 1-tail). For olaparib alone, the median PFS was 8.2 months (HR, 1.20; 95% CI, 0.93-1.54), and for standard-of-care chemotherapy, the median PFS was 10.3 months.
“The challenge with cediranib and olaparib is the tolerance of cediranib. In addition, this combination with the widespread use of olaparib earlier on for treatment will most likely not be widely accepted.”
Hematologic Malignancies
Saad Z. Usmani, MD

“The ECOG trial and SWOG trials were perhaps the most impactful, even though both did not meet their primary endpoints,” Saad Z. Usmani, MD, chief of the Plasma Cell Disorders Program and director of Clinical Research in Hematologic Malignancies at the Levine Cancer Institute, Atrium Health told Targeted Oncology. “The ECOG trial demonstrated no benefit of KRD over VRD for standard-risk multiple myeloma whereas the SWOG trial demonstrated no benefit of adding [elotuzumab] to RVD induction/maintenance for high-risk multiple myeloma.”
PFS Data Show Carfilzomib Triplet Remains Inferior to VRd in Newly Diagnosed Myeloma
The next-generation proteasome inhibitor carfilzomib (Kyprolis) in combination with lenalidomide (Revlimid), and dexamethasone (KRd) failed to improve PFS in patients with newly diagnosed multiple myeloma compared with the standard-of-care, bortezomib (Velcade) plus lenalidomide and dexamethasone (VRd) in the ENDURANCE (E1A11) study.
The Addition of Elotuzumab to RVd Fails to Improve Patient Outcomes in High-Risk Myeloma
Elotuzumab (Empliciti) in combination with bortezomib, lenalidomide, and dexamethasone (RVd) did not improve patient outcomes, but the PFS and OS in both study arms exceeded original statistical assumptions(NCT01668719). The median PFS with the combination regimen was 31 months compared with 34 months with RVd (HR, 0.968; 80% CI, 0.697-1.344; P = .449), and the median OS was 68 months versus not reached, respectively (HR, 1.279; 80% CI, 0.819-2.000; P = .478).4
Other Impactful Data in Hematology
“The other impressive studies were the BCMA-directed CAR T and bispecific monoclonal antibody trials, specifically the JNJ-4528 and teclistamab. Both showed excellent response rates and safety profiles in [patients with] advanced relapsed/refractory multiple myeloma,” said Usmani.
Teclistamab Appears Safe, Efficacious in Relapsed/Refractory Multiple Myeloma
As patients with relapsed/refractory multiple myeloma tend to have poor prognoses when they run out of treatment options, teclistamab (JNJ-64007957) appeared to be a safe and efficacious treatment approach in a phase I study (NCT03145181) of this patient population.
The primary objective of the dose-escalation portion of the study was determining the doses for part 2, and the doses ranged from 0.3-720 µg/kg weekly. There was a 67% ORR (50% ≥very good partial response) at 270 µg/kg. The drug became active in patients receiving 38.4 µg/kg or higher, with 21 out of 52 patients (38%) achieving a response.
Efficacy data at the 720-µg/kg dose are not yet mature, and the dose escalation and expansion phases of the trial are still ongoing, Usmani explained in his presentation during the meeting. However, the future findings could lay the groundwork for a promising new therapy for these patients.
Deep and Durable Responses Still Observed With JNJ-4528 in Relapsed/Refractory Myeloma
The chimeric antigen receptor (CAR) T-cell therapy JNJ-4528 showed continued deep and durable responses as treatment of patients with relapsed/refractory myeloma in the phase 1b/2 CARTITUDE-1 (NCT03548207) study. The ORR was 100% with a stringent CR rate of 86%. The PFS rate was 86% at 9 months with this therapy as well (95% CI, 67%-95%).
No new safety signals were observed in this study. In particular, cytokine release syndrome was common, occurring in 93% of the patients, although only 7% of patients experienced the event in grade 3 severity or greater. Neurotoxicity, another common toxicity of CAR T cells, was observed in 10% of patients, with 3% of patients experiencing grade 3 or greater neurotoxicity.
The phase 1b portion of the study aimed to determine the safety of BCMA and confirm a dose for the phase 2 portion, which will investigate the efficacy of the treatment in terms of ORR.
Breast Cancers
Erika Hamilton, MD

The addition of tucatinib (Tukysa) to trastuzumab (Herceptin) plus capecitabine resulted in more than a 60% reduction in the risk of CNS progression or death in both patients with previously treated HER2-positive metastatic breast cancer who have active or stable brain metastases, according to findings from the HER2CLIMB study (NCT02614794).
“The updated ASCO analysis of HER2CLIMB showed that among patients with brain metastases, both PFS and OS were improved with the addition of tucatinib to capecitabine and trastuzumab,” said Erika Hamilton, MD, director, Breast Cancer and Gynecologic Cancer Research Program, Sarah Cannon Research Institute. “The subset of patients with treated and stable brain metastases as well as the subset with untreated asymptomatic and treated and progressive brain metastases also benefitted with longer time without progression when they received tucatinib. This gives us confidence in using this regimen both for our patients with and without brain metastases,” said Hamilton.
Frontline Pembrolizumab Plus Chemo Shows Compelling PFS Improvement in PD-L1+ TNBC
Frontline pembrolizumab in combination with multiple chemotherapy agents induced a statistically significant and clinically meaningful improvement in PFS as treatment of patients with metastatic triple-negative breast cancer (TNBC) who have high PD-L1 expression compared with chemotherapy alone in the phase 3 KEYNOTE-355 trial (NCT02819518).
“KEYNOTE-355 echoed the results of our previous trial IMpassion 130 with atezolizumab,” Hamilton said. “In KEYNOTE-355, patients receiving first line chemotherapy were randomized to also receive pembrolizumab or placebo. Similar to IMpassion130, KEYNOTE was able to identify roughly 40% of [patients with] TNBC with high PD-L1–expressing tumors who remained progression free for longer with the addition of pembrolizumab.”
The combination had a median PFS of 9.7 months compared with 5.6 months for chemotherapy alone, which translated to a 35% reduction in the risk of disease progression or death (HR, 0.65; 95% CI, 0.49-0.86; one-sided P = .0012).
“This solidifies the ability for immunotherapy to add efficacy to a PD-L1 high subset in 1st line metastatic triple negative breast cancer,” Hamilton concluded.
Stephanie Graff, MD

Long-Term Data Confirms Role of Genomic Testing in HR-Positive Breast Cancer
The primary end point of distant metastasis free survival (DMFS) in patients with chemotherapy-untreated clinical high/genomic low risk patients with breast cancer continued to be met, according to the long-term results of the phase 3 MINDACT study (NCT00433589) of the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy. These results confirmed MINDACT as a positive de-escalation study.5
“In HR-positive disease, we also had an update from MINDACT looking at the Amsterdam 70-gene assay, which shows that at 8.7 years of follow up the assay still has significant prognostic value and can help identify patients who can safely omit chemotherapy,” Stephanie Graff, MD, director, Breast Cancer Program and Clinical Research, Sarah Cannon Cancer Institute, HCA Midwest Health, told Targeted Oncology. “This further solidifies the role of genomic profiles as a standard tool to appropriately select chemotherapy in early stage HR-positive breast cancer, particularly those who are lymph node–negative or with low-volume lymph node–positive disease.”
Biomarker Data Confirm Phase 3 KATHERINE Study Findings of T-DM1 in HER2+ Breast Cancer
Exploratory analyses of the KATHERINE study (NCT01772472) provided the first data on the relationship between biomarker expression after HER2-targeted therapy and invasive DFS. The invasive DFS benefit observed was consistent across all biomarker subgroups for trastuzumab emtansine compared with trastuzumab. These results demonstrated that the PI3K mutational status did not influence outcomes for treatment with either trastuzumab or T-DM1.6
According to Graff, the KATHERINE study was another important update. “The KATHERINE investigators [analyzed] tissue markers like PI3K mutations, PD-L1, and degree of Her2 expression showing that the benefit continues to persist in all groups. This continues to confirm the practice-changing results of the KATHERINE trial.”
The benefit of T-DM1 was maintained in patients with high HER2 expression, which suggests this treatment may be able to overcome resistance mechanisms compared with trastuzumab.
Reference
- ASCO Virtual Scientific Meeting Convened Record Number of Oncology Professionals from across the globe. News release. ASCO. June 4, 2020. https://bit.ly/2XxZBtF. Accessed June 4, 2020.
- Wainberg ZA, Fuchs CS, Tabernero J, et al. Efficacy of pembrolizumab (pembro) monotherapy versus chemotherapy for PD-L1–positive (CPS ≥10) advanced G/GEJ cancer in the phase II KEYNOTE-059 (cohort 1) and phase III KEYNOTE-061 and KEYNOTE-062 studies. J Clin Oncol 38, 2020 (suppl 4; abstr 427).
- Bendell JC, Strauss J, Fahik M, et al. Phase I monotherapy dose escalation of RGX-202, a first-in-class oral inhibitor of the SLC6a8/CKB pathway, in patients with advanced gastrointestinal (GI) solid tumors. J Clin Oncol 2020;38(suppl):3504. doi:10.1200/JCO.2020.38.15_suppl.3504
- Usmani SZ, Ailawadhi S, Sexton R, et al. Primary analysis of the randomized phase II trial of bortezomib, lenalidomide, dexamthasone with/without elotuzumab for newly diagnosed, high-risk multiple myeloma (SWOG-1211). J Clin Oncol 2020;38(suppl):8507. doi:10.1200/JCO.2020.38.15_suppl.8507
- Cardoso F, van ’t Veer L, Pncet C, et al. MINDACT: Long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J Clin Oncol 2020;38(suppl):506. doi:10.1200/JCO.2020.38.15_suppl.506
- Denkert C, Lambertini C, Fasching PA, et al. Biomarker data from KATHERINE: a phase III study of adjuvant trastuxzumab emtansine (T-DM1) versus trastuzumab (H) in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer. J Clin Oncol 2020;38(suppl):502. doi:10.1200/JCO.2020.38.15_suppl.502