Where Monoclonal Antibodies and CAR T-cell Therapy Fit in R/R DLBCL
At a live, virtual event, Farrukh Awan, MD, provided commentary on the current treatment algorithm for patients with relapsed/refractory diffuse large B-cell lymphoma and where the use of the combination regimen tafasitamab and lenalidomide fits in.
At a live, virtual event, Farrukh Awan, MD, provided commentary on the current treatment algorithm for patients with relapsed/refractory diffuse large B-cell lymphoma and where the use of the combination regimen tafasitamab (Monjuvi) and lenalidomide (Revlimid) fits in as physicians continue to see successes with chimeric antigen receptor T-cell therapy. Awan also discussed how to handle toxicities from the combination approach and how he dose-reduces in patients. Further, he discusses the future of the space for patients if they need fixed-duration or indefinite-duration treatment.
Treatment Algorithm in the Relapsed/ Refractory Setting
There are lots of debates [when it comes to the treatment algorithm], and this is just 1 opinion.... Some people prefer to do things a certain way, which they feel more comfortable with, but for the most part, I think we all agree that CAR [chimeric antigen receptor] T-cell therapy is here to stay. At least for the near future, unless something else comes up, but for now, I think we all agree that CAR T-cell [therapy] in this second-line space is [now] an established therapy, and also in the third-line space, where it was first approved.1 We do have a proportion of patients we can cure, and depending on what data you look at, across the board we are probably looking at 30% to 40% cure rates. So if you were to think about the large cell lymphoma space, there is debate in the frontline setting, [such as] with using polatuzumab vedotin [Polivy] in that setting about whether we should use polatuzumab plus rituximab [Rituxan] with cyclophosphamide, doxorubicin, and prednisone [R-CHP], or whether we should continue to use R-CHOP [rituximab, cyclophosphamide, hydroxydaunorubicin hydrochloride, vincristine, and prednisone].2 If we move beyond that, what is important right now is that...patients who get R-CHOP chemotherapy, and then relapse within a year after therapy, those patients tend to have the worst outcomes. So that’s a cohort of people who have high-risk disease and that’s where the [use of] CAR T-cell therapies [was] studied and recently approved in the second-line setting...and we have 2 products that are approved: One is axicabtagene ciloleucel [Yescarta] and the other one is lisocabtagene maraleucel [Breyanzi].3,4
For most of us who are taking care of a lot of [patients with] large cell lymphoma, if we have a patient who is progressing on or failed R-CHOP those are the patients we take to [treatment with] CAR T-cell therapy.5 Next is those patients who relapsed beyond 1 year then got salvage therapy, and those are the patients who will now be considered for transplant or CAR T-cell therapies.
Those are the 2 big distinctions we are making right now in the field, [if the patient relapsed in] less than 1 year or more than 1 year. If you have a patient who relapsed at month 13 or 11, you can go either way.5 A lot of times, what makes this tricky is we have patients who finished R-CHOP therapy and by the time they get the repeat scan, 3 months down the road or more, they have progressive disease. The disease is progressing so fast that people end up starting them on salvage chemotherapy. Interestingly, some of those patients have chemotherapy-sensitive disease and how you deal with those patients is the big question.
If that patient goes into a complete remission after 2 cycles of R-ICE [rituximab, ifosfamide, carboplatin, and etoposide] then should you take them to autologous stem cell transplant [ASCT] or should you not take them to ASCT? You know, these are all questions that are very opinion-driven right now but these are topics that come up all the time. We keep discussing it because people are trying to address [this challenge] in multiple different ways.... [Ultimately, we are asking ourselves] how do we position [treatment sequencing] around CAR T-cell therapy and around transplant? And how do we tease it apart?
5-Year Updated L-MIND Data
[The overall response rate (ORR) on the L-MIND trial (NCT02399085)] was 57% [95% CI, 45.9%-68.5%] and we saw a higher response rate in patients who had 1 prior line of therapy [at 67.5% (95% CI, 45.9%-79.4%)], as expected, but then as you get into 2 or more prior lines of therapy, the ORR goes down [to 47.5% (95% CI, 31.5%-63.9%)]6....
More prior lines of therapy means you’re dealing with a much more aggressive disease, but this is follow-up after 5 years. So I think that’s something to be said about [the combination of] tafasitamab and lenalidomide. Complete response, which again is a big marker, [was also seen] in a substantial percentage of patients [and seen in] more than 50% in the 1 prior line of therapy [group].6
In terms of duration of response [DOR], there was some stability, but beyond the 2- and 3-year marks [DOR] seems to plateau. Now is that [because some patients were] cured? Because patients are on maintenance therapy beyond 3 to 4 years we need more [cases to assess] and we need a little bit longer follow-up. Still, at this point, this is a 5-year follow-up, and that’s impressive. We are seeing some disease stability with ongoing treatment, and it’s promising that [the treatment is] impacting the cancer and trying to keep it under control.6
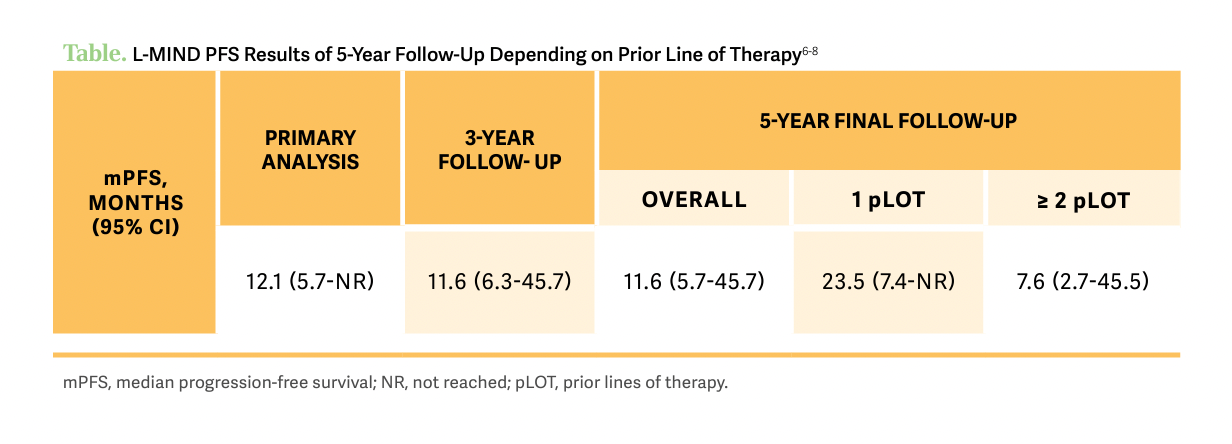
[Now], if you break down [progression-free survival] by line of therapy, as expected, if the patient was less heavily pretreated, they were doing much better than patients who had more treatments in the past [Table6-8]. This is why this is provocative and important to note that there is some stability, especially after the first couple of years [of treatment] and patients don’t tend to relapse often if they are continued on [the combination therapy]. Similarly, if the patient is in remission, their survival is improved, and if they had 1 prior line of therapy those patients have better outcomes. All in all, these are very promising results with ongoing therapy with tafasitamab and lenalidomide.
Handling the Safety Profile of Tafasitamab and Lenalidomide
Now, if you are going to keep somebody on treatment for a long time, if you have a toxic treatment then that would not be attractive for patients who will have issues. Fortunately, the adverse event [AE] profile of this treatment has been pretty good, so far6.... In this setting, the most frequent AEs that we expect are infections and cytopenias; those historically have [shown up quite a bit]. [On this study], the bottom line is that beyond 2 years we saw few grade 3 and 4 AEs and we mostly saw mild AEs with tafasitimab.6
[Looking at these treatment-emergent AEs in detail], as expected, cytopenias are the most common problem in these patients, [with] neutropenia and thrombocytopenia [being the most common events per person over the 5 year follow-up, with 67 events of thrombocytopenia and 167 events of neutropenia] and then infections, which historically have been the issue.6 Most of the problems are happening [when treatment is combined with lenalidomide or just lenalidomide] in the first 12 months of therapy, as expected.6 In a lot of these patients [who need treatment] beyond first- or second-line of therapy, every time I use lenalidomide I end up dose-reducing because of neutropenia. That’s been a big problem with [the addition of] lenalidomide, but as [the patient] gets off it their immune system recovers and the frequency of [neutropenia] goes down substantially. This is why this was an option for indefinite therapy. It is well tolerated [and can be modified], so there is some justification for using this approach, which can potentially control disease for a long period of time.
Looking at Decisions for Long-Term Treatment
We do see durable responses [with this combination], but all those responses happen when patients continue therapy. The big question is now whether this is curing the cancer, and I don’t know if anybody will say that it’s curing it. Having said that, if [the patient] had a small amount of disease and if you drop therapy and don’t have the cancer come back, that would be our answer. But without having a head-to-head trial with CAR T-cell therapy or stopping therapy and showing the same remissions, it’s hard to say that it’s curative. So at this point, 2 to 3 years is the minimum [amount of treatment] I would prefer to do. After that, it’s an ongoing discussion, that if the patient is in a complete metabolic response, I will feel much better in stopping [treatment], otherwise, I would probably continue [tafasitamab and lenalidomide], but you could argue [that’s when you could stop it, too]. So these are perfect questions that we have no answer to currently.
Initially, [when it comes to how the patient’s age changes their treatment]... I said, “I’m not going to treat anybody above 75 [with this],” and then after 6 months I went to 77 years of age, because I had a patient who was 77. And then I went up to 80, then 82, and right now my oldest patient in our center is 84 years old. I’ve heard somewhere that 87- to 88-year-olds have gone through CAR T-cell therapy.
In the last 6 months, when I went on my inpatient rotation...[almost] every single patient on CAR T-cell therapy was about 75 or 77 years old. So this is a major advantage that as we get more comfortable with managing CAR T-cell [therapy] we have pushed it to the point that we can get [older and frailer] patients through it without too much toxicity, and the majority of patients do well. In my personal experience, anytime I’ve used these treatments after CAR T-cell therapy, it doesn’t seem to work as well. [When I use it] before CAR T-cell therapy, I get the most benefit out of it.
But the question [still] comes up, especially in unlimited-duration therapy, of when do you stop and when do you progress to the next step? So that’s the issue that I’ve been dealing with and that’s why I have not necessarily seen a lot of responses after CAR T-cell failures.
REFERENCES:
1. FDA approves new treatment for adults with relapsed or refractory large-B-cell lymphoma. News release. US Food and Drug Administration. February 5, 2021. Accessed September 15, 2023. https://tinyurl.com/2tyue9xt
2. Thomas C, Thapa S, McLaughlin C, et al. Point and counterpoint: polatuzumab vedotin in the front-line therapy for diffuse large B- cell lymphoma. Front Oncol. 2023;12:1098375. doi:10.3389/fonc.2022.1098375
3. FDA approves axicabtagene ciloleucel for second-line treatment of large B-cell lymphoma. US Food and Drug Administration. April 1, 2022. Accessed September 14, 2023. https://tinyurl.com/53dye236
4. FDA approves lisocabtagene maraleucel for second-line treatment of large B-cell lymphoma. US Food and Drug Administration. June 27, 2022. Accessed September 15, 2023. https://tinyurl.com/5n8zyr4c
5. Westin J, Sehn LH. CAR T cells as a second-line therapy for large B-cell lymphoma: a paradigm shift? Blood. 2022;139(18):2737-2746. doi:10.1182/blood.2022015789
6. Duell J, Abrisqueta P, Andre M, et al. Five-year efficacy and safety of tafasitamab in patients with relapsed or refractory DLBCL: Final results from the phase II L-MIND study. Presented at: American Association for Cancer Research Annual Meeting 2023; April 14-19, 2023; Orlando, FL. Accessed May 12, 2023. https://bit.ly/3MmShus
7. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
8. Duell J, Maddocks KJ, González-Barca E et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. doi:10.3324/haematol.2020.275958

Examining the Non-Hodgkin Lymphoma Treatment Paradigm
July 15th 2022In season 3, episode 6 of Targeted Talks, Yazan Samhouri, MD, discusses the exciting new agents for the treatment of non-Hodgkin lymphoma, the clinical trials that support their use, and hopes for the future of treatment.
Listen
What Is Dark Zone Lymphoma, and Is It Clinically Relevant?
January 16th 2025Dark zone lymphoma includes aggressive B-cell lymphomas with shared molecular features. While some respond to escalated treatment, others remain resistant, highlighting the need for targeted approaches to improve outcomes.
Read More