Tipifarnib Yields 100% Disease Control in HRAS-Mutant Head and Neck Squamous Cell Carcinoma
The signal transduction inhibitor tipifarnib induced disease control in all patients with HRAS-mutant head and neck squamous cell carcinoma and a high variant allele frequency, according to preliminary results of a phase II trial presented at the 2019 American Association for Cancer Research–National Cancer Institute–European Organization for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics.
Alan L. Ho, MD, PhD

Alan L. Ho, MD, PhD
The signal transduction inhibitor (STI) tipifarnib induced disease control in all patients withHRAS-mutant head and neck squamous cell carcinoma (HNSCC) and a high variant allele frequency (VAF), according to preliminary results of a phase II trial presented at the 2019 American Association for Cancer ResearchNational Cancer Institute–European Organization for Research and Treatment of Cancer International Conference on Molecular Targets and Cancer Therapeutics.1
“We have compelling antitumor activity in the heavily pretreated cohort of patients with recurrent metastatic head and neck cancer withHRASmutations,”Alan L. Ho, MD, PhD, Geoffrey Beene Junior Faculty Chair at Memorial Sloan Kettering Cancer Center in New York, New York, said during a presentation of the data. “This occurred regardless of previous progression on chemotherapy, immunotherapy, and cetuximab.”
Ho said that preclinical evidence suggesting thatHRASmutations are not thought to be particularly susceptible to certain biological mechanisms that allowNRASandKRASto circumvent STI action served as the rationale for the trial.
Tipifarnib is an agent that inhibits the farnesyltransferase enzyme. According to Ho, STIs were developed over 2 decades ago as a biologically rational way to target oncogenicRASmutations in human malignancies. That was based on the observation that mutantRASneeds to localize to the plasma membrane to initiate oncogenic activation; farnesyltransferase catalyzes a key step in the process and was thought to be a therapeutic vulnerability that would allow targetingRAS.
“Unfortunately, the initial clinical trials that were done that presumably were enriched forRAS-mutant tumors or solid tumors did not show significant activity at that time for the development of STIs or cancer therapeutics,” Ho said. “We were enthusiastic to develop this trial, which was meant to test the hypothesis that clinicallyHRAS-mutant solid tumors are more susceptible to STI action or tipifarnib.”
Patients withHRAS-mutant solid tumors with measurable disease by RECIST 1.1 and no curative therapies available were eligible to be treated in this study. Patients must have had an ECOG performance status of ≤1 and were assigned to 1 of 2 cohorts: Those with thyroid cancer were in cohort 1; cohort 2 included patients with any other solid tumor type but was later amended to just HNSCC because of the significant signals observed in this population.
Initial dosing on this trial was 900 mg of oral tipifarnib twice daily on days 1 through 7 and days 15 through 21 every 28 days. In the first 17 patients withHRAS-mutant HNSCC, rapid and durable responses were seen despite resistance to chemotherapy, cetuximab (Erbitux), or immunotherapy in previous lines of treatment. A subset of patients also needed dose reductions, and by the end of cycle 1, the median dose that patients received was reduced to 600 mg twice daily. When investigators looked at treated patients withHRASmutations, most major responders were those with allelic frequency of ≥20%, with those exhibiting frequencies of ≥35% experiencing a greater enrichment of response.
Based on these observations, the trial was amended further to include 30 patients with HNSCC, and enrollment was limited to those with anHRASVAF of ≥20% and serum albumin ≥3.5 g/dL orHRASVAF ≥35%.
A third patient cohort was added so investigators could look at tipifarnib in the setting of squamous cell carcinoma other than HNSCC.2
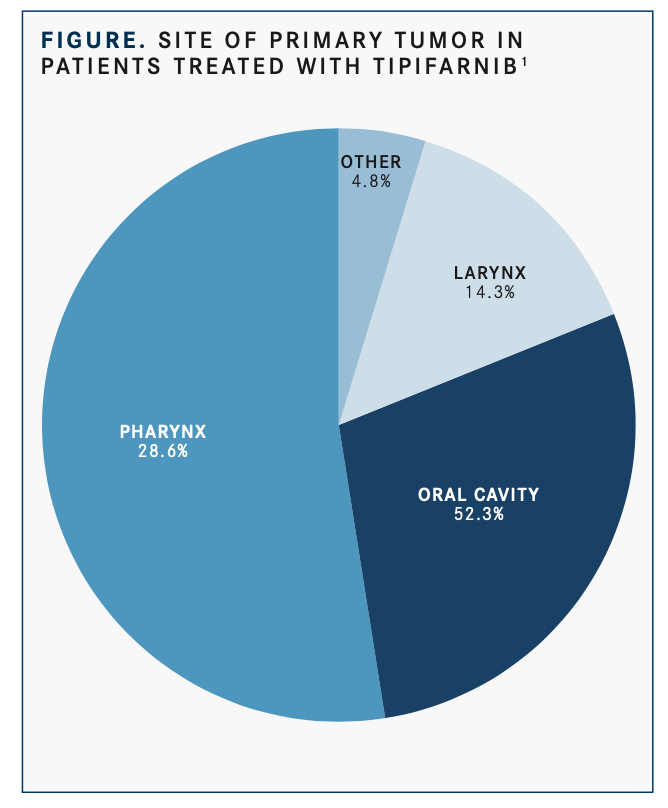
In the 21 total treated patients with HNSCC, the median age was 64 years, 66.7% were men, the median number of prior anti- cancer regimens was 2, and the site of the primary tumor was the oral cavity in about half (52.4%;FIGURE).1All but 2 patients had received prior platinum (90.5%), 13 (61.9%), immunotherapy; and 11 (52.4%), cetuximab.
Among 18 evaluable patients with high VAF, 10 had an objective response (56%; 95% CI, 0.31-0.78). The other 8 treated patients had stable disease, 2 of which had unconfirmed partial responses.
Among patients with a response, the median progression-free survival (PFS) was 8.3 months compared with 4.5 months among those with stable disease.
“What is noteworthy here is that in this very heavily pretreated patient population, none had progression of disease as their best response,” Ho said. “Many of these responses were quite durable...with 4 of the major responses lasting over a year and 6 lasting over 6 months.”
Some patients with a shorter duration of response remain on tipifarnib therapy, he noted. “To put this into greater context, in the same population of patients [for whom] nivolumab [Opdivo] and pembrolizumab [Keytruda] were approved in the platinum- refractory setting, those response rates ranged from 13% to 15%. In those phase III trials, the standard-of-care arm with chemotherapy or cetuximab yielded a response rate of <10%.”
Treatment-emergent adverse events were consistent with the known safety profile of tipifarnib, with the majority of grade ≥3 events being hematologic and managed with best supportive care.
According to Ho, these data resulted in the initiation of the international, multicenter, open-label, phase II AIM-HN/SEQ-HN pivotal study assessing the safety and efficacy of tipifarnib inHRAS-mutant HNSCC.3 This noncomparative trial will have a primary end point of objective response rate (ORR) and include 2 cohorts. The first will assess the ORR of tipifarnib in patients withHRASmutations. The second, an observational substudy, includes 2 groups of patients: One group will have participants from the initial cohort and for whom there are available data; the other, matched control patients with HNSCC and wild-typeHRASwho consent to provide first-line outcome data and additional follow-up. Secondary end points include duration of response, time to response, PFS, and overall survival.
“We are exploring opportunities to combine tipifarnib with immunotherapy, chemotherapy, or targeted agents, particularly in those patients who have <20%HRASVAF,” Ho concluded.
References
- Ho AL, Brana I, Haddad R, et al. Preliminary results from a phase 2 trial of tipifarnib in head and neck squamous cell carcinomas (HN-SCC) with HRAS mutations. Presented at: American Association for Cancer Research.
- Ho AL, Chau N, Bauman J, et al. Preliminary results from a phase II trial of tipifarnib in squamous cell carcinomas (SCCs) with HRAS mutations.Ann Oncol.2018;29(suppl 8; 1046O). doi: 10.1093/annonc/mdy287.002.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More