The Emerging Role of Checkpoint Inhibitors in Lymphoid Malignancies
In this review, our authors discuss the mechanism of action and clinical development of various checkpoint inhibitors in lymphoma.
Introduction
Lymphoma is the most common hematologic malignancy in the United States, with an estimated 81,000 new cases of Hodgkin (HL) and non-Hodgkin lymphoma (NHL) combined and 21,000 deaths in 2015.1NHL is the sixth most common type of cancer in both males and females, accounting for 5% and 4% of new cancer cases, respectively.1Survival rates for both HL and NHL have improved significantly over the past several decades; however, outcomes continue to be poor in patients with relapsed/ refractory disease.2Recently, the introduction of novel targeted agents has ushered in a new era in management of lymphomas, especially in the relapsed/refractory setting and in frail, elderly patients with multiple comorbid conditions who are unable to tolerate cytotoxic combination chemotherapy and presents an interesting field for further research.
The immune system plays a key role in the surveillance, detection, and elimination of cancer cells. The curative role of immune therapy has long been recognized in patients with hematologic malignancies, with a prime example being allogeneic stem-cell transplantation. Immune checkpoints refer to a plethora of inhibitory pathways hardwired into the immune system that are crucial for maintaining self-tolerance. In addition, they modulate the duration and amplitude of physiological immune responses in peripheral tissues in order to minimize collateral tissue damage.3Checkpoint inhibitors act by "releasing-the-brakes" on the patient’s immune system rather than targeting the tumor directly, and have demonstrated clinically meaningful results in various solid malignancies (ie, melanoma, renal cell carcinoma, lung cancer). This therapeutic strategy emerged from the recognition that tumors can evade the host immune system by usurping immune checkpoint pathways such as the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed-death 1 (PD-1) pathways. In this review, we discuss the mechanism of action and clinical development of various checkpoint inhibitors in lymphoma.
Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4)
CTLA-4, the first immune checkpoint receptor to be clinically targeted, is expressed exclusively on T cells and primarily regulates the amplitude of the early stages of T cell activation.3 CTLA-4 downregulates T cell activity and leads to a decrease in the antitumor immune response.4-6The significance of CTLA-4 in maintaining normal immunological homeostasis was confirmed by the findings that CLTA-4 deficient mice die from fatal lymphoproliferative disorders.7Blocking CTLA-4 therefore promotes the persistence and activation of intratumoral T cells.8
Primarily CTLA-4 counteracts the activity of the T-cell constimulatory receptor CD28. CD28 and CTLA-4 share identical ligands: CD80 (also known as B7-1) and CD86 (also known as B7-2). CD28 does not affect T-cell activation unless the T cell receptor (TCR) is first engaged by a cognate antigen. Once antigen recognition occurs, CD28 signaling strongly amplifies TCR signaling to activate T cells.3Since CTLA-4 has a much higher overall affinity for both ligands, its expression on the surface of T cells dampens the activation of T cells by outcompeting CD28 in binding CD80 and CD86, as well as actively delivering inhibitory signals to the T cells.4-6CTLA-4 also confers "signaling-independent" Tcell inhibition through the sequestration of CD80 and CD86 from CD28 engagement, as well as active removal of CD80 and CD86 from the antigen-presenting cell (APC) surface.9
Programmed-Death Pathway
Programmed-death (PD-1, CD 279) is a member of the B7 receptor family, expressed by activated T cells, activated B cells, natural killer cells (NK), and myeloid cells. PD-1 expression is induced when T cells become activated.3It plays a major role in regulation of immune responses by interacting with 2 ligands, programmed death ligand 1 (PD-L1) (B7-H1 or CD274) and programmed death ligand 2 (PD-L2) (B7-DC or CD273).10PD-L1 is expressed by B and T cells and macrophages, mediating a generalized anti-inflammatory effect; whereas PD-L2 is expressed by APC10 and regulates T-cell priming.11 These ligands are upregulated by the inflammatory environment and inhibit function of PD-1-bearing lymphocytes. When engaged by one of its ligands, PD-1 inhibits kinases that are involved in T-cell activation through the phosphatase SHP2. In a healthy host, PD-1/PD-L1 signaling regulates effector T-cell responses and protects bystander tissues from immune-mediated damage. This pathway is harnessed by many tumors to evade immune surveillance and forms a major resistance mechanism within the tumor microenvironment.
Mice deficient in PD-1 have an immune phenotype distinct from mice deficient in CTLA-4, as CTLA-4 is believed to primarily regulate early T-cell activation and PD-1 is believed to inhibit T-cell effector activity in the effector phase.12The inhibition of T-cell activity by PD-1 engagement appears stronger than by CTLA-4 engagement, although the phenotype of PD-1 knockout mice is less severe than that of CTLA-4 knockout.13The expression of PD-1 is also observed on other immune subsets including T-regulatory (Treg) cells, B cells, and NK cells; Treg cells heavily infiltrate many tumors and suppress effector immune responses, thus PD-1 blockage may also increase antitumor cytotoxicity through increased NK-cell killing and reduction in Treg cell number and function.114,15PD-1 binds to its ligands PD-L1 and PD-L2 and the interaction results in downstream inhibitory signals resulting in apoptosis of activated T cells.16Chronic antigen exposure, such as in chronic viral infection or cancer, can lead to persistently high PD-1 expression, which is associated with T-cell exhaustion; blockade of the PD-1/ PD-ligand pathway augmented or restored the function of viral-infection specific and tumor-specific CD4+ and CD8+ T cells in mouse and human studies.3
Expression of PD-1 and Its Ligands in Lymphomas
Lymphomas variably express PD-1 and its ligands.17PD-1 expression is common among tumor cells in chronic lymphocytic leukemia (CLL) and angioimmunoblastic T-cell lymphoma (AITL) but rare in other NHL subtypes.18,19PD-1+ T cells are frequently demonstrated in rosettes surrounding Reed-Sternberg (RS) cells in both classical Hodgkin lymphoma (cHL) and nodular lymphocyte subtypes.119,20PDL1 expression is common in tumor cells in HL and primary mediastinal B-cell lymphoma (PMBL) where it appears to be associated with amplification in chromosome 9p24.121 and aggressive virus-driven lymphomas.22A large proportion of cHL tumors have increased surface expression of PD-L1,22 strongly suggesting that HL may have a genetic dependence on the PD-1 pathway for survival. HL is characterized by the presence of few RS cells surrounded by an extensive, but ineffective immune infiltrate. Amplification of 9p24.1 is a recurrent genetic abnormality in HL and mediates PD-L1 and PD-L2 expression.21PD-1 ligand expression is also increased through the JAK2/STAT pathway, as the extended 9p24.1 amplification region also includes the JAK2 locus.21In addition, EBV infection, seen commonly in HL also increases PD-1 ligand expression.23PD-L1 is also expressed on diffuse large B-cell lymphoma (DLBCL) cells and tumor-infiltrating non-malignant cells, primarily macrophages.17,22Andorsky and colleagues studied PD-L1 expression and functional activity in cell lines and lymphoma specimens.17PD-L1 was expressed uniformly in anaplastic large cell lymphoma (ALCL) cell lines, but rarely in B-cell NHL. PDL-1 expression in B-cell lymphoma was observed in activated B-cell (ABC) DLBCL. Anti-PD-L1 blocking antibody boosted proliferation and IFN-γ secretion by allogeneic T cells responding to ALCL and DLBCL cells.17
The prognostic implication of PD-1 and its ligand expression on tumor cells in lymphoma is conflicting. In 2 studies, a high proportion of follicular lymphoma (FL) cells expressing PD-1 correlated with a favorable overall survival (OS).20,21In other reports, the prognostic impact of PD-1 expression was negligible or adverse.22 PD-1 is expressed on tumor-infiltrating lymphocytes (TILs) in DLBCL, and the presence of a large number of PD-1+ TILs is associated with favorable OS in patients with DLBCL.23,24 In a prospective cohort of DLBCL patients treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R+CHOP), elevated soluble PD-L1 was adversely prognostic for OS.24In a recent study describing the clinicopathological impact of PD-L1+ DLBCL, Kiyasu et al defined a new sub-entity termed microenvironmental PD-L1+ (mPD-L11) DLBCL (ie, PD-L1DLBCL in which PD-L1+ nonmalignant cells are abundant in the tumor microenvironment).25 The prevalence rates of PD-L1+ and mPD-L1+ DLBCL were 11% and 15.3%, respectively. Both PD-L1+ and mPD-L1+ DLBCL were significantly associated with non-germinal center B-cell (GCB) type and Epstein-Barr virus positivity. Patients with PD-L1+ DLBCL had inferior OS compared with patients with PD-L1– DLBCL. In contrast, there was no significant difference in OS between mPD-L1+ and mPDL1–DLBCL.25
In summary, level of expression of PD-1 and its ligands may be used to guide early development of checkpoint inhibitors in various lymphoma subtypes; however, it is not ready for primetime as either a predictor of response or prognosis.
Monoclonal Antibodies Targeting the CTLA-4 Axis in Lymphoma
Ipilimumab
Ipilimumab is a recombinant human IgG1 monoclonal antibody that binds to CTLA-4 and enhances T-cell activation and proliferation. It has significant clinical benefit in solid tumors such as melanoma, and it is approved as frontline therapy in metastatic melanoma.26A phase I study of ipilimumab was performed in patients with relapsed and refractory B-cell NHL.27Eighteen patients [14 follicular lymphoma (FL), 3 DLBCL, 1 mantle cell lymphoma (MCL)] were treated with ipilimumab at 2 dose levels with an 11% (2/18) overall response rate (ORR). One DLBCL patient achieved a complete response (CR) and 1 FL patient achieved a partial response (PR). Despite low rates of response, the responses were durable for both patients lasting 31 months for the DLBCL patient and 19 months for the FL patient. Ipilimumab was well tolerated, with common adverse events being diarrhea, headache, abdominal pain, anorexia, fatigue, neutropenia, and thrombocytopenia. Correlative studies confirmed that T-cell proliferation to recall antigens was increased in a significant proportion of patients, suggesting that the immune response was activated and enhanced.
Ipilimumab was also studied in the post-allogeneic stem cell transplant (alloSCT) setting in hematological malignancies.28Twenty-nine patients with hematological malignancies that were recurrent or progressive after alloSCT received a single infusion of ipilimumab; responses were predominantly seen in patients with lymphoma. There were 2 CR in patients with HL and a PR in a patient with MCL. Interestingly, ipilimumab did not induce or exacerbate clinical graft versus host disease or graft rejection in this study. There is currently a phase I trial investigating the combination of ipilimumab and rituximab in patients with relapsed or refractory B-cell lymphoma (NCT01729806).
Monoclonal Antibodies Targeting the PD-1 Axis in Lymphoma
Nivolumab
Nivolumab is a fully human IgG4 monoclonal antibody that inhibits PD-1 activity by binding to the PD-1 receptor and blocking its interaction with its ligands PD-L1 and PD-L2. It has shown promising results in the treatment of solid tumors and is currently approved by the FDA for treatment of advanced/metastatic melanoma and metastatic non small-cell lung cancer. A phase I study of nivolumab in patients with relapsed/refractory hematologic malignancies (NCT01592370) including classical HL, B-NHL, T-NHL, and multiple myeloma is currently underway. Results for the 23 patients with HL were published earlier this year.29
The HL study consisted of a high risk population with a median of 4 to 5 lines of prior systemic therapy with 78% of patients relapsing post brentuximab vedotin and 78% of patients relapsing post-autologous stem cell transplant (ASCT). Patients received nivolumab 3 mg/kg every 2 weeks until CR, tumor progression, or intolerable toxicity. ORR was 87% (20/23) with 17% CR and 70% PR rate. The remaining 3 patients had stable disease. The rate of progression-free survival (PFS) at 24 weeks was 86%. Many of the responses appeared durable with some patients in continued remission for over a year, though the follow-up time of this study was short. Nivolumab was well tolerated in the study population. Drug-related adverse events of any grade and of grade 3 occurred in 78% and 22% of patients, respectively. No grade 4 or 5 events were reported. The most common adverse events were rash (22%) and decreased platelet count (17%). Drug-related grade 3 events reported in 5 patients consisted of myelodysplastic syndrome, pancreatitis, pneumonitis, stomatitis, increased lipase levels, decreased lymphocyte count, and leucopenia.
Nivolumab was granted FDA "Breakthrough Therapy" designation for HL. A phase II study of nivolumab in patients with classical HL who have relapsed post-ASCT has recently completed enrollment (NCT02181738). There is considerable interest in introducing nivolumab in the frontline setting in HL; however, to our knowledge, no such clinical trials have been initiated.
Results for the remaining lymphoid malignancies have been reported in an abstract form.30Twenty-nine patients with B-NHL, 2 patients with PMBL, and 23 patients with T-NHL were enrolled. These patients were heavily pretreated with more than two-thirds of patients receiving ≥ 3 prior therapies. The most common drug-related serious adverse event was pneumonitis (7%). The ORR for patients with B-NHL was 28% (7% CR) with an ORR of 36% in patients with DLBCL, and 40% in patients with FL. The ORR was 17% in patients with T-cell NHL (no CR) with an ORR of 40% in the 5 patients with peripheral T-cell lymphoma. These results have led to 2 additional phase II clinical trials investigating the efficacy of nivolumab in relapsed or refractory FL (NCT02038946) and relapsed or refractory DLBCL (NCT02038933), which are ongoing.
Pembrolizumab
Pembrolizumab is a humanized IgG4 anti-PD-1 monoclonal antibody that also blocks binding of PD-1 to its ligands PD-L1 and PD-L2. Since IgG4 cannot engage the Fc receptor and cause antibody-dependent cellular cytotoxicity (ADCC) of PD-1+ cells, enhancement of antitumor immune response is the primary mechanism of action.
Pembrolizumab was approved by the FDA for treatment of advanced/metastatic melanoma and nonsmall-cell lung cancer.
The KEYNOTE-013 trial is a phase Ib study investigating the safety and efficacy in patients with relapsed or refractory hematologic malignancies (NCT01953692). Results of the classical HL cohort of this study were presented at the American Society of Hematology (ASH) 2014 conference in an abstract form.31Twenty-nine patients with relapsed/ refractory HL received pembrolizumab 10 mg/kg every 2 weeks. More than half the patients had received at least 5 prior lines of therapy; all had received brentuximab vedotin and two-thirds had undergone ASCT. ORR was 66% (all PRs) in the whole cohort, 75% in patients relapsing post-ASCT, and 44% in patients who did not receive ASCT. Median duration of response was not reached. The most common drug-related adverse events were grade 12 respiratory events (20%) and thyroid disorders (20%). Grade 3 or higher treatment-related adverse events were rare and included axillary pain, hypoxia, joint swelling, and pneumonitis in 1 patient each. There were no grade 4 events or treatment-related deaths. The trial is still in progress with final results including data from the B-NHL arm still pending. A phase II study of pembrolizumab in relapsed/refractory classical HL is currently recruiting (KEYNOTE-087, NCT02453594).
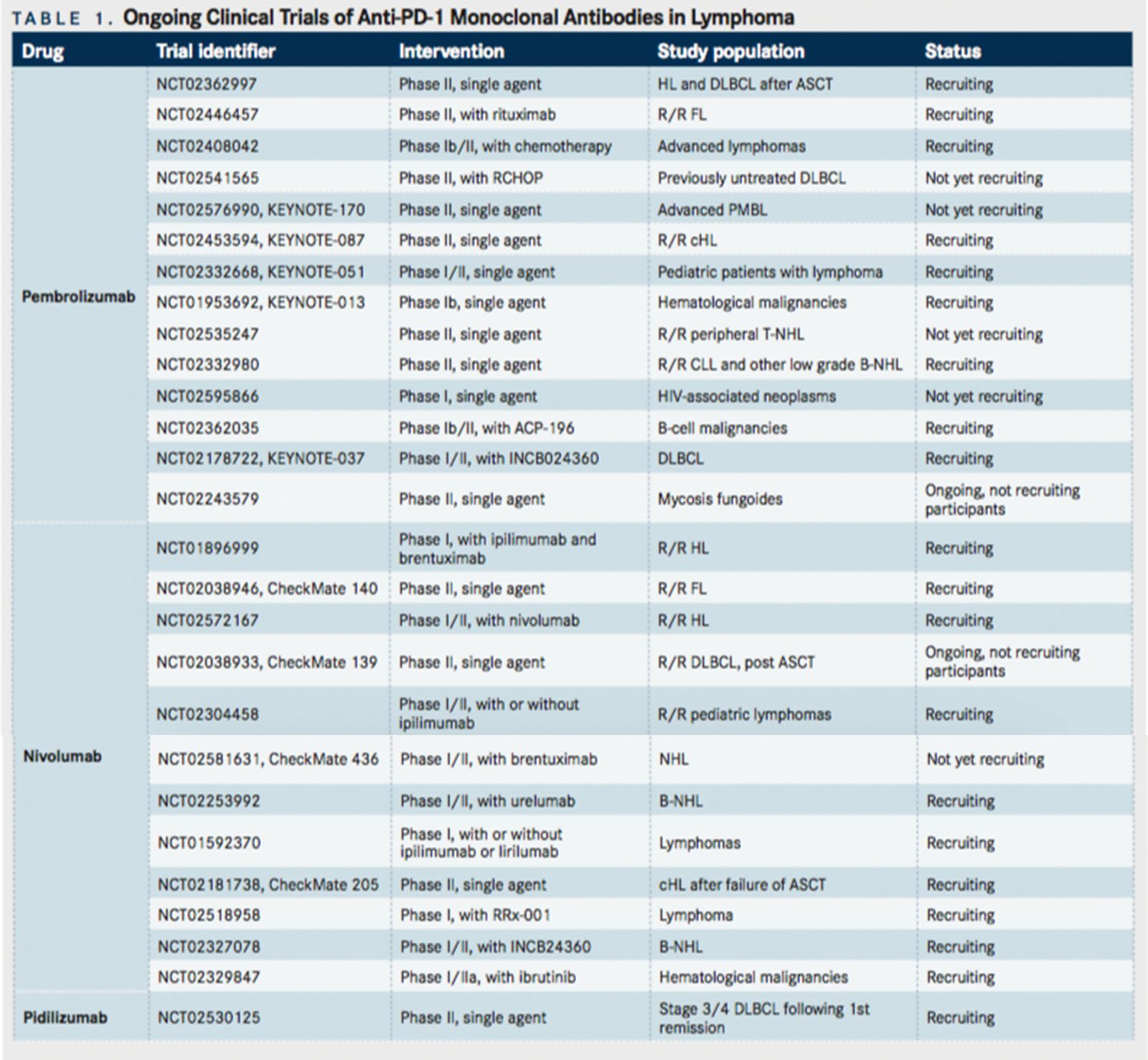
Pembrolizumab is currently under intensive investigation in a variety of settings in lymphoma, as summarized inTABLE 1. Studies are also investigating the efficacy of pembrolizumab as consolidation therapy post ASCT in DLBCL and HL (NCT02362997), in combination with rituximab in relapsed FL (NCT02446457), and in combination with chemotherapy for advanced lymphoma (NCT02408042).
Pidilizumab (CT-011)
Pidilizumab is an anti-PD-1 humanized IgG1 monoclonal antibody that blocks PD-1 activity, leading to stimulation of NK-cell activity and extended effector/memory T-cell survival. These changes are associated with the enhancement of antitumor immune responses and the generation of tumorspecific memory cells.32
The first in-human study was a phase I trial conducted by Berger et al. Seventeen patients with advanced hematologic malignancies (CLL-3, DLBCL-2, HL-1, FL-1) were treated with a single dose of pidilizumab at 5 dose levels ranging from 0.2 to 6 mg/kg.32The treatment was well tolerated and no dose-limiting toxicity was observed. Six patients (35%, including 1 FL, 2 CLL and 1 HL) had clinical benefit, with 1 CR in a patient with FL that lasted over a year. Based on this encouraging efficacy signal, pidilizumab has been moved into phase II trials in lymphoid malignancies.
Armand et al conducted an international phase II study of pidilizumab in patients with DLBCL or PMBL after ASCT.33The rationale for selecting this population was the expression of PD-L1 in a subset of patients, low tumor burden post-ASCT, and remodeling of the immune system in the post-transplant setting. There was a preponderance of NK cells, monocytes, and CD45RO+ memory/effector cells among the circulating lymphocytes post ASCT, which comprise pidilizumab target populations and portend a favorable prognosis. Sixty-six patients were treated with 3 doses of pidilizumab 1.5 mg/kg beginning 1 to 3 months after ASCT. The 16-month PFS was 72%. Of note, the 18-month PFS was 70% among the 24 patients who had a positive positron emission tomography scan after pre-ASCT salvage therapy. These results compared favorably to the 52% PFS in an otherwise similarly high-risk historical control population. Among 35 patients with measurable disease post-ASCT, CR and ORR were 34% and 51%, respectively. The safety profile was favorable with no apparent autoimmune toxicity, treatment-related deaths, or infusion reactions. The main non-hematologic adverse events were fatigue, upper respiratory tract infection, diarrhea, cough, and hyperglycemia. Grade 3-4 neutropenia and thrombocytopenia occurred in 14 (20%) and 6 (8%) patients, respectively. Treatment with pidilizumab was associated with an increase in PD-L1-bearing activated helper T cells and PD-1 ligand bearing monocytes, suggesting an on-target in vivo effect of pidilizumab.
Westin et al performed a single center phase II study of pidilizumab with rituximab in patients with relapsed follicular lymphoma.34Thirty-two patients with rituximabsensitive FL relapsing after 1 to 4 previous therapies were treated with 4 doses of pidilizumab 3 mg/kg every 4 weeks, with an option to receive up to an additional 8 doses for a total of 12 infusions. Rituximab was given 375 mg/m2 weekly for 4 doses commencing 17 days after the first dose of pidilizumab. Median follow-up was 15.4 months. In the 29 response-evaluable patients, ORR was 66% with 52% CR and median PFS of 18.8 months. The combination was well tolerated with no grade 3-4 adverse events. The most common grade 1 events were anemia and fatigue, with respiratory infection being the most common grade 2 adverse event. Responders expressed higher levels of PD-L1 on peripheral blood T cells and monocytes at baseline compared to non-responders. The in vivo on-target effect of pidilizumab was demonstrated by increased expression of activation-associated genes by T cells and NK cells in day 14 samples compared to baseline.
A phase II study of pidilizumab in patients with stage III-IV DLBCL following first remission is currently recruiting participants (NCT02530125).
In summary, anti-PD-1 antibodies have shown variable response in lymphomas ranging from an impressive 87% ORR in HL to 17% in T-cell lymphomas (for nivolumab). This underlines the inherent immunologic heterogeneity of lymphomas with the immunogenic tumors such as cHL responding much more briskly than other lymphoma subtypes. The safety profiles of nivolumab, pembrolizumab, and pidilizumab have been favorable. Most patients experienced mild drug-related adverse events with no treatmentrelated deaths. The frequencies of immune-related adverse events (irAEs) commonly noted with anti-PD-1 therapy were lower compared to previously reported trials in solid tumors [nivolumab29: rash-22%, grade 3/4 none; diarrhea-13%, grade 3/4 – none; hypothyroidism – 9%, grade 3/4 – none; pancreatitis grade 3/4 – 4%; pembrolizumab 31: grade 1-2 respiratory events – 20%; grade 1-2 thyroid disorders – 20%, grade 3/4 irAEs – none; pidilizumab 33,34: serious irAEs – none], however, since most lymphoma trials are phase I, data are immature, with most results being available in only abstract form, and the patient numbers are inadequate to comment on this issue conclusively.
Agents in Early Clinical Development
Agents Targeting PD-1
Several agents targeting the PD-1 axis are currently under investigation; although many have shown promising results in solid malignancies, their clinical development in lymphomas is in preliminary stages.
- AMP-514 (MEDI0680) is an anti-PD-1 monoclonal antibody (mAb) currently in phase I trials both as a single agent and in combination with the IgG1 antiPD-L1 mAb MEDI4736 in advanced malignancies (NCT02118337).
- Preclinical data on the PD-L2-Ig fusion protein AMP224 suggests additional activity beyond PD-1 ligand antagonism.35It may have synergistic activity with vaccine approaches and low-dose cyclophosphamide, and is under evaluation in a phase I study (primarily in patients with advanced melanoma) combined with low-dose cyclophosphamide.36
- The branched amino-acid peptide sequence AUNP-12 is engineered from the PD-1/PD-L2 binding sequence and appears to have promising preclinical activity, but is yet to enter clinical trials to our knowledge.37
Agents Targeting PD-L1
- Durvalumab (MEDI4736) is a fully human IgG1 antiPD-L1 monoclonal antibody that is currently under investigation in a phase Ib study in combination with tremelimumab or AZD9150 in patients with relapsed/ refractory DLBCL (NCT02549651). It is also being studied in combination with ibrutinib in relapsed/ refractory DLBCL and FL (NCT02401048).
- MPDL3280A is a humanized IgG1 anti-PD-L1 monoclonal antibody that is currently being tested in phase I/II trials in combination with obinutuzumab in patients with relapsed/refractory FL and DLBCL (NCT02220842).
Multiple additional immune checkpoints are promising targets for therapeutic blockade based on preclinical experiments, and inhibitors for many of these are under active development:
- Varlilumab is a fully human anti-CD27 mAb that is currently under phase I trials in hematological malignancies. Favorable, although preliminary reports of clinical activity in lymphoma have been presented in abstract form.38 No dose-limiting toxicity was reached in a cohort of 13 B-NHL patients (3 HL, 3 FL, 3 marginal zone lymphom [MZL], 4 DLBCL). One patient with HL who had previously progressed following treatment with chemotherapy, autologous stem cell transplant, and brentuximab vedotin had a PR with 77% shrinkage in measurable disease; treatment is ongoing. Three additional patients had stable disease (range: 4.5 to 11+ months).
- Lymphocyte-activation gene 3 (LAG-3, CD223) is another immune checkpoint that is expressed on activated T cells, NK cells, B cells, and plasmacytoid dendritic cells. BMS-986016 is an anti-LAG3 mAb that is currently in phase I clinical trials in hematological malignancies (NCT02061761).
- CD137, a positive regulator of T-cell function, is also targetable using the agonist mAb urelumab, which is in trial for NHL (NCT01775631 and NCT01471210).
As our knowledge of immune checkpoints expands, a plethora of agents are entering clinical investigation and it is likely that at least a few will reach the clinic over the next few years (See ).
Future Directions
Immunotherapy is revolutionizing the practice of oncology, but we have just scratched the surface of this vastly complicated biological machinery. Much needs to be done in order to deepen our understanding of the immune system so that more efficacious agents with a better toxicity profile can be developed.
- Identification of appropriate patients. The first step is to determine which tumors to target and in what setting. To that effect, today there are a very large number of ongoing, planned, and proposed trials with a host of newer agents, both as single-agents and in combination. These trials will generate a plethora of data that needs to be carefully scrutinized in order to gain maximum traction from this treatment modality.
- Defining predictive biomarkers. PD-L1 expression has emerged as a potential predictive biomarker for PD-1-directed therapy. Multiple, distinct, companion assays for PD-L1 positivity have been developed, but there is as yet no comparison, standardization, or prospective validation of these assays. PD-L1 expression on tumor cells and/or the tumor-immune infiltrate is likely only part of the predictive model necessary for selecting patients predisposed to respond to monotherapy. Additional predictive biomarkers are necessary to identify patients most likely to benefit from PD-1-based combination therapy, since tumor cell PD-L1 expression appears to have limited predictive value in this setting.39
- Specifically, the utility of PD-L1 expression as a response predictor in lymphomas is unknown as the clinical trials reported so far have focused on change in immune subsets in the peripheral blood33 or gene expression profiling on paired tumor biopsies.34Patients with markers of good antitumor immune responses in vivo may benefit to a large extent from anti-PD-1 therapy.34This was clearly evident in the phase II study of pidilizumab and rituximab in follicular lymphoma, where expression of PD-L1 on peripheral blood CD4+ CD8+ and CD14+ cells was higher in responders than nonresponders. By gene expression profiling, high expression of a 41-gene Teff signature was associated with superior PFS in patients treated with pidilizumab and rituximab (but not in patients treated with chemotherapy). Because the expression of PD-L1 on DLBCL cells may be restricted to a subset of tumors, it may be that future selection of patients for PD-1 blockade on the basis of ligand expression in the tumor or microenvironment could lead to a greater clinical benefit in the appropriate patient subgroups.
- Endpoint choice and response assessment. Current response assessment in lymphomas as per the Lugano classification for lymphoma is optimized for conventional cytotoxic therapy.40 However, it has been proven in several trials in solid malignancies that patients who would have been deemed "treatment failures" (early progression/stable disease) based on conventional imaging criteria, may eventually derive durable benefit from immunotherapy. Hence, there is a need to define immune-related response criteria, as without them, we may not recognize therapeutically valuable results.
- Development of combination therapies. Developing rational combinations of checkpoint inhibitors with cytotoxic chemotherapy, targeted agents, other checkpoint inhibitors, and immunotherapeutic agents is an active area of interest. The hypothesis behind combining cytotoxic therapy and checkpoint inhibitors is that in addition to cytoreduction, cytotoxic agents can cause "immunogenic" apoptosis by releasing tumor antigens at the site of the tumor, thus enhancing the activity of agents targeting the PD-1 axis.13,41This benefit has to be balanced against the potential immunosuppression caused by chemotherapy. Some cytotoxic therapies such as anthracyclines or ionizing radiation are associated with release of danger molecules from tumor cells such as calreticulin, high-mobility group box protein B1 (HMGB1), and ATP, which can polarize dendritic cells (DCs) towards a pro-inflammatory phenotype and increase priming towards Th1 anti-tumor T cells and away from Tregs.42Additionally, some chemotherapies such as cyclophosphamide are directly toxic to immunosuppressive Tregs, and combining low-dose cyclophosphamide with tumor vaccines induces anti-tumor immunity in animal models.43Similarly, gemcitabine can selectively kill myeloid-derived suppressor cells (MDSCs) in vitro and in vivo, and animal models show that gemcitabine substantially increases the activity of immunotherapy when the two are given in combination.44Targeted cancer therapies [such as vascular endothelial growth factor (VEGF)VEGF receptor inhibitors, RAF inhibitors, antibodies targeted to receptor tyrosine kinases that are overexpressed in tumors, and epigenetic therapies] that are not conventionally thought of as immunotherapies can elicit or enhance antitumor immunity. These therapies may therefore force the tumors to upregulate immune checkpoints that consequently can be blocked as part of a combinatorial strategy.41The other option for combination therapy is to combine checkpoint blockade therapy with other types of immunotherapy, including cellular immunotherapies such as chimeric antigen receptor (CAR) T cells, tumor vaccines, or oncolytic viral therapy. Combinations with agents that allow better presentation of tumor antigens by APC (antiCD40 agonists, vaccines, interferon-α, and toll-like receptor agonists), those that increase priming and activation of T-lymphocytes (anti-CTLA4, anti-OX40) or infiltration of T cells into tumors (anti VEGF) seem promising. A small trial combining autologous granulocyte macrophage colony stimulating factor (GM-CSF) secreting tumor cell vaccines with CTLA4 blockade found increased inflammatory infiltrates and tumor regression26, suggesting that vaccineinduced anti-tumor T cells were present within the tumor but anergized due to CTLA4 co-inhibition. Similarly, in a pre-clinical study combining vaccines and PD1 blockade, mice receiving combination therapy had increased overall survival and decreased tumor growth.25Additionally, combining blockade of multiple inhibitory pathways decreasesT-cell anergy and improves T-cell responsiveness. In one pre-clinical study, animals treated with cancer vaccines were found to have significantly higher overall survival if they were concurrently treated with antibodies to PD1 and CTLA4 compared to animals treated with vaccination and either antibody alone. Combining different checkpoint inhibitors has already shown clinical benefit in melanoma,45and there is hope to replicate this success in lymphomas. This approach works by preventing resistance of tumors to escape immunosurveillance by alternate pathways. Two ongoing phase I trials, one testing the combination of nivolumab and ipilimumab (NCT01592370), and another testing the combination of nivolumab and urelumab, include an NHL cohort (NCT02253992). Trials are also ongoing combining anti-PD-1 antibodies with ibrutinib (NCT02329847, NCT02401048) and anti-CD20 antibody, obinutuzumab (NCT02220842). The vast numbers of possible combinations far exceeds the capability to test them all in clinical trials the choice will likely be driven by the immunological characteristics of the individual tumor and the patient. Immunogenic tumors (such as cHL) may be best suited to immunostimulatory combinations such as PD-1/PD-L1 blockage and ipilimumab. In contrast, immunological inert lymphomas might be best treated with chimeric antigen receptor (CAR) T cells in combination with anti-CD20 monoclonal antibodies.46
Conclusion
Treatment with immune checkpoint inhibitors has promising clinical activity in lymphoma, particularly in patients with HL. However, responses are not uniform across various histologies of lymphoma, and further research is needed to understand the differences in the biology of both the "drug" and the "disease." With a plethora of ongoing, planned, and proposed trials, clinical testing is already outpacing our understanding of the underlying checkpoint biology. Careful scrutiny of generated data and continued collaboration between bench and bedside will maximize the therapeutic potential of this revolutionary strategy.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
- Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184-4190.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264.
- Hathcock KS, Laszlo G, Dickler HB, et al. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262(5135):905-907.
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182(2):459-465.
- Alegre ML, Frauwirth KA, Thompson CB. T cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1(3):220-228.
- Tivol EA, Boyd SD, McKeon S, et al. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4- deficient mice. J Immun. 1997;158(11):5091-5094.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734-1736.
- Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600-603.
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580-6587.
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430-439.
- Armand P. Immune checkpoint blockade in hematologic malignancies. Blood. 2015;125(22):3393-3400.
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015-3029.
- Terme M, Ullrich E, Aymeric L, et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393-5399.
- Seo SK, Seo HM, Jeong HY, et al. Co-inhibitory role of T cell-associated B7-H1 and B7-DC in the T cell immune response. Immunol Lett. 2006;102(2):222-228.
- Andorsky DJ, Yamada RE, Said J, et al. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor- associated T cells. Clin Cancer Res. 2011;17(13):4232-4244.
- Xerri L, Chetaille B, Serriari N, et al. Programmed death 1 is a marker of angioimmunoblastic T cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39(7):1050-1058.
- Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T cell lymphoma. Am J Surg Pathol. 2006;30(7):802- 810.
- Nam-Cha SH, Roncador G, Sanchez-Verde L, et al. PD-1, a follicular T cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol. 2008;32(8):1252-1257.
- Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268-3277.
- Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462-3473.
- Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611-1618.
- Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367-2375.
- Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193-2201.
- Prieto PA, Yang JC, Sherry RM, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039-2047.
- Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446-6453.
- Bashey A, Medina B, Corringham S, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581-1588.
- Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319.
- Lesokhin AM, Ansell SM, Armand P, et al. Preliminary results of a phase I study of nivolumab (BMS-936558) in patients with relapsed or refractory lymphoid malignancies. Blood. 2014;124(21):291-312.
- Moskowitz CH, Ribrag V, Michot JM, et al. Blockade with the monoclonal antibody pembrolizumab (MK-3475) in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: preliminary results from a phase 1b study (KEYNOTE-013). ASH Meeting Abstracts. 2014;124(21):290.
- Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044-3051.
- Armand P, Nagler A, Weller EA, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199-4206.
- Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69-77.
- Mkrtichyan M, Najjar YG, Raulfs EC, et al. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J Immunol. 2012;189(5):2338-2347.
- Infante JR, Powderly JD, Burris HA, et al. Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. ASCO Meeting Abstracts. 2013;31(Suppl 15):3044.
- Sasikumar PG, Satyam LK, Shrimali R, et al. Abstract 1231: Equipotent antagonism, transient immune activation and excellent antitumor efficacy with a peptide inhibitor of PD-1 immune check point pathway. Cancer Research. 2013;73(Suppl 8):1231.
- Ansell S, Northfelt D, Flinn I, et al. A phase I study of an agonist anti- CD27 human antibody (CDX-1127) in patients with advanced hematologic malignancies or solid tumors. J Immunother Cancer. 2013;1(Suppl 1):P259.
- Mahoney KM, Atkins MB. Prognostic and predictive markers for the new immunotherapies. Oncology (Williston Park). 2014;28 Suppl 3:39-48.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
- Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237-251.
- Zitvogel L, Apetoh L, Ghiringhelli F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118(6):1991-2001.
- Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336-344.
- Suzuki E, Kapoor V, Jassar AS, et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713-6721.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122-133.