Single-Dose Netupitant/Palonosetron Bests 3-Day Aprepitant-Based Regimen to Prevent CINV
A single-dose combination of netupitant/palonosetron demonstrated better efficacy compared with a 3-day aprepitant-based regimen, according to results of a pooled analysis of multiple phase 2/3 studies investigating the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy.

A single-dose combination of netupitant/palonosetron (NEPA) demonstrated better efficacy compared with a 3-day aprepitant-based regimen (APR), according to results of a pooled analysis of multiple phase 2/3 studies investigating the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Findings were presented at the 2020 National Comprehensive Cancer Network Virtual Annual Conference by investigators led by Rudolph Navari, MD, PhD, of the University of Alabama in Birmingham.1
NEPA was also more effective than the 3-day APR in preventing significant nausea—which is the most difficult-to-control symptom with cisplatin-based HEC—in the overall phase (0 to 120 hours) following chemotherapy.
In the delayed phase (25 to 120 hours), breakthrough CINV on individual days differed between treatment groups. Generally, NEPA rates decreased each day with significantly lower rates of breakthrough CINV and breakthrough nausea on days 3 through 5, and the APR regimen stayed at a relatively constant rate each day.
“This may be related to the longer half-life of netupitant (80 hours) compared to aprepitant (9 to 13 hours),” the investigators wrote in their conclusion.
Three pivotal trials were evaluated for this analysis, all of which were multicenter, randomized, double-blind/double-dummy, and registration studies of cisplatin-based HEC.2-4 The 1197 patients included on these trials received a single oral dose of NEPA plus dexamethasone before chemotherapy on day 1 (n = 621) or a 3-day regimen of APR comparator/control treatment (n = 576). All of the patients received dexamethasone on days 2 through 4.
The complete responses, no significant nausea, and complete protections were all similar for patients in the acute phase (0 to 24 hours) with P values of .649, .074, and .231, respectively.
However, these efficacy end points showed significant differences of response rates in the delayed phase for patients on NEPA plus dexamethasone and those on APR, 5-HT3RA, and dexamethasone. There were 81.8% of patients with complete responses on NEPA versus 76.9% of patients on APR (P .038). The response rates for complete protection in the delayed phase were 73.6% in the NEPA group and 68.4% in the APR group (P =.049). Patients with no significant nausea had a rate of 81.5% with NEPA and 76.4% with APR (P =.032).
The overall phase response rates for complete response and complete protection were not statistically significant, with P values of .163 and .061, respectively. For patients with no significant nausea in the overall phase, there were response rates of 79.5% for those receiving NEPA and 74.1% for those receiving APR (P =.027).
The number of patients treated with NEPA who experienced breakthrough CINV and significant breakthrough nausea on days 3 through 5 following chemotherapy was considerably fewer than those treated with APR.
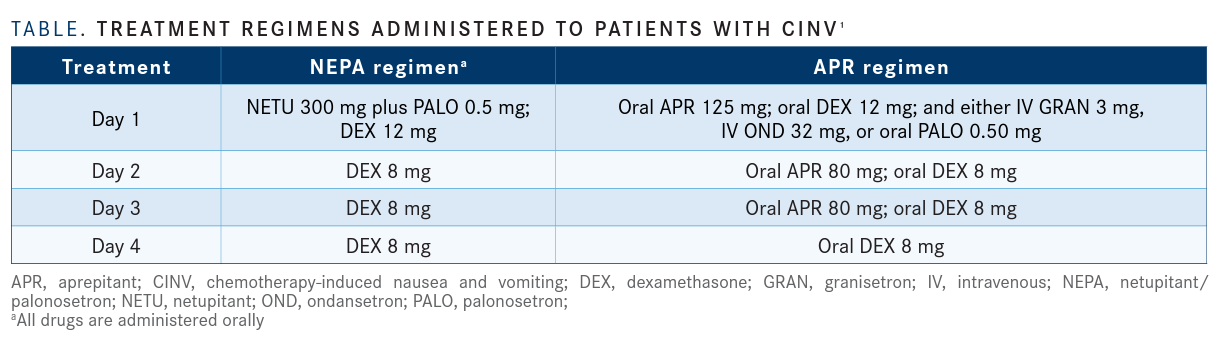
For the treatment group, patients received netupitant at 300 mg, palonosetron at 0.50 mg, and 12 mg of oral dexamethasone on day 1, followed by 8 mg of dexamethasone on days 2 through 4. For the control group, patients received oral APR at 125 mg; granisetron at 3 mg intravenously, ondansetron at 32 mg intravenously, or oral palonosetron at 0.50 mg; and oral dexamethasone at 12 mg on day 1. The control group also received oral APR at 80 mg on days 2 and 3 and oral dexamethasone at 8 mg on days 2 through 4 (TABLE).1
Regarding baseline characteristics across the studies, 67% were male patients, and the mean age was 55 years. Patients with lung cancer, which was the most common cancer type, made up 56% of the study population. Other cancer types in the studies were head and neck and ovarian. Other notable characteristics included race—67.8% in the NEPA arm and 72.9% in the APR arm were Asian, and the rest were white—and cisplatin dose, with a median of 73.6 mg/m2 (range, 27.5-200) for patients on APR and 74.7 mg/m2 (range, 25.8200) for patients on NEPA.
To keep track of emetic episodes, severity of nausea, and rescue medication, patients in all of the studies completed a diary from day 1 of chemotherapy to day 5. Nausea severity was self-rated on a 100-mm horizontal visual analog scale which ranged from 0 mm, meaning they had no nausea, to 100 mm, meaning the nausea was as bad as it could be.
The efficacy end points for this post hoc analysis included patients with complete response (no emesis and/or no rescue medication), no significant nausea (<25-mm maximum nausea severity score on the 100-mm scale), complete protection (no emesis, no rescue medication, and no significant nausea), and breakthrough CINV (the opposite of complete response or complete protection or the presence of significant nausea).
The treatments and associated risks were compared for each end point for the acute, delayed, and overall intervals. Each individual day from day 2 to day 5 post-chemotherapy were compared as well, using generalized linear models with loglink function and binomial distribution.
References:
1. Navari R, Bonizzoni E, Wickham R, et al. Pooled analysis of phase 3 studies comparing a single-dose fixed combination of netupitant/ palonosetron (NEPA) vs a 3-day aprepitant-based regimen (APR) for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Poster presented at: 2020 NCCN Virtual Annual Conference; March 19-22, 2020.
2. Hesketh PJ, Rossi G, Rizzi G, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25(7):1340-1346. doi:10.1093/annonc/mdu110
3. Gralla RJ, Bosnjak SM, Hontsa A, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25(7):13331339. doi:10.1093/annonc/mdu096
4. Zhang L, Lu S, Feng J, et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol. 2018;29(2):452-458. doi:10.1093/annonc/mdx698
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More