RDI and Ammonia Levels May Predict Tolerability of Lenvatinib in HCC
Good responses to lenvatinib were observed in patients with hepatocellular carcinoma who maintained a high relative dose intensity of 75% or greater.

Good responses to lenvatinib (Lenvima) were observed in patients with hepatocellular carcinoma (HCC) who maintained a high relative dose intensity (RDI) of 75% or greater, according to a poster presented during The Liver Meeting Digital Experience, the American Association for the Study of Liver Diseases’ annual conference held virtually in November. Patients with RDI of 75% or greater demonstrated a progression-free survival rate of 9.1 months (95% CI, 5.0-not evaluable) compared with 5.9 months in patients with an RDI of less than 75% (95% CI, 3.9-9.8, P =.3045).
RDI is used to measure the total dose of chemotherapy and is defined as the actual dose received divided by the standard calculated dose during a set period. In the poster, Taniki et al analyzed the background factors of 92 patients who achieved an RDI ≥75% and 3 patients who discontinued treatment due to adverse effects.
The factors included age, body weight, body mass index, and ECOG performance status. Also reviewed were the liver function tests aspartate aminotransferase (AST), alanine transaminase (ALT), albumin, and total bilirubin. Further laboratory results examined levels of sodium, ammonia, prothrombin time, platelet count, presence of ascites, and hepatic encephalopathy.
Determining the maximum tolerable dose for systemic therapy agents is affected by liver function tests. Further stratifying patients according to liver function helps promote optimal treatment.
The median age of patients in the retrospective study was 71.7 years, and 87% were male. The majority of patients had ECOG performance status 0 (48.9%) or 1 (37.0%). Regarding staging, 69.6% were Child-Pugh A, 30.4% were Child-Pugh B, and the majority (72.8%) had advanced disease as determined by Barcelona Clinic Liver Cancer staging.
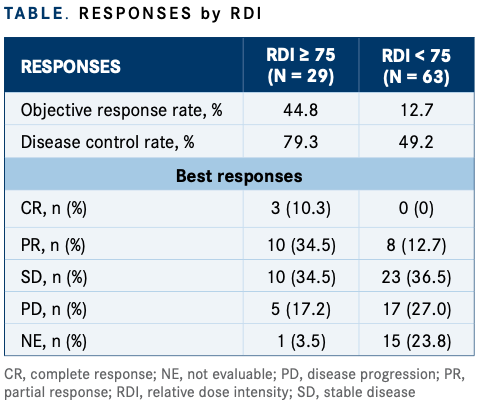
Objective response rates in the RDI 75 or greater group (n = 29) were 44.8% versus 12.7% in the RDI less than 75 group (n = 63). The disease control rate was 79.3% versus 49.2%, respectively, according to the investigators (TABLE).
The investigators conducted a multivariate analysis of RDI and found that age and ammonia levels had statistical significance. Median age in the RDI 75 or greater group was 67.4 years (± 6.3) and in the RDI less than 75 group, median age was 73.6 years (± 10.1) (multivariate odds ratio, 0.92; 95% CI, 0.85-0.99; univariate P = .0039). Ammonia levels in the RDI 75 or greater group was 32.9 μg/dl (± 12.2) versus 53.8 μg/dl (± 33.0) in the RDI less than 75 group (multivariate odds ratio, 0.94; 95% CI, 0.90-0.99; univariate P = .0142).
Among patients who developed intolerance to lenvatinib (n = 26), median ammonia levels were 62.7 μg/dl (+/– 37.5) compared with 41.3 μg/dl (+/– 23.8) in patients who were tolerant of lenvatinib (n = 66; multivariate odds ratio,1.02; 95% CI, 1.00-1.04; P = .0106). Median percentage of ascites in the intolerant group was 53.9% compared with 16.7% in the tolerant group (multivariate odds ratio, 6.16; 95% CI, 1.24-30.5; P = .0005).
Differences in albumin and bilirubin levels were statistically significant in the intolerant versus the tolerant groups. Investigators observed a median albumin level of 3.4 g/dl (+/– 0.4) in the intolerant group compared with 3.8 g/dl (± 0.5) (P = .0020). Median total bilirubin was 1.09 mg/dl (± 0.48) in the intolerant group versus 0.81 mg/dl in the tolerant group (± 0.44) (P = .0084).
Adverse effects were similar between the RDI 75 and greater and RDI less than 75 groups, according to investigators. In the overall cohort, common adverse events (grade 3 or greater) were decreased appetite (5.4%), proteinuria (5.4%), decreased liver function (5.4%), and diarrhea (4.3%). The investigators reported that 28.3% of patients in the overall cohort discontinued treatment because of adverse effects.
In the RDI 75 or greater group, common adverse effects (grade 3 or greater) were hand-foot skin reaction, decreased appetite, diarrhea, and increased bilirubin. These were reported in 3.4% of patients. Discontinuation of treatment was reported in 20.7% of patients.
In the RDI less than 75 group, common adverse effects (grade 3 or greater) were decreased liver function and proteinuria (both were seen in 7.9% of patients), decreased appetite (6.3%), and hypertension (4.8%). Discontinuation of treatment was reported in 31.7% of patients.
The investigators noted that patients who had discontinued treatment because of adverse effects did not have overt hepatic encephalopathy; however, serum ammonia levels were significantly related to both RDI and treatment discontinuation. They concluded that serum ammonia level may be useful for evaluating liver function and predicting tolerability of lenvatinib treatment.
REFERENCE:
Taniki N, Nakamoto N, Chu PS, et al. Analysis of factors related to the tolerability of lenvatinib for patients with unresectable hepatocellular carcinoma in real-world conditions: a multicenter study. Presented at: American Association for the Study of Liver Diseases, The Liver Meeting Digital Experience; November 13-16, 2020; virtual. Abstract 1141. Accessed January 12, 2021. https://bit.ly/2LrNonh
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More