PD-L1 as a Biomarker Predictive of Response to Checkpoint Inhibition in Lung Cancer
A review of data regarding PD-L1 expression as a predictive biomarker of response to checkpoint inhibition in lung cancer.
Abstract
The advent of immunotherapy has changed the treatment landscape of many solid tumors, amongst which, non-small cell lung cancer (NSCLC). While reports of impressive efficacy and favorable side effect profiles are numerous, little is known about which patients should be selected to receive immunotherapy. The activation of the programmed death-1 (PD-1) and programmed-death ligand-1 (PD-L1) axis is known to cause tumor regression via T-cell activation. The presence and level of PD-L1 in tumor tissues has been postulated by many to serve as a biomarker, predictive of response to checkpoint inhibition. Here, we review the data as it relates to PD-L1 expression in lung cancer and its use as a predictive biomarker.
Introduction
1
Lung cancer is the leading cause of cancer mortality in the US, projected to lead to over 158,000 deaths in 2016.Cytotoxic chemotherapy has been the cornerstone in the treatment of advanced non-small cell lung cancer (NSCLC). Eventual treatment failure or discontinuation due to toxicity is nearly guaranteed and much research into alternative treatment paradigms has taken place.
In recent years, we have witnessed many advances in the treatment of advanced NSCLC. The identification and characterization of aberrant signalling pathways paved the way for targeted therapy using tyrosine kinase inhibitors. Unfortunately, only a small proportion of patients stand to benefit from such treatments, as actionable mutations are present in only a minority of tumor cells. Recently, immune checkpoint inhibition has led to exciting treatment advances in many solid tumors, including NSCLC.
2
Targeting of the programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) pathways with nivolumab or pembrolizumab, respectively, have led to the approval of both agents in multiple malignancies. Activation of the PD-1/PD-L1 signalling axis leads to inhibition and even depletion of the tumor-infiltrating T-lymphocytes.Inhibition of these pathways has led to durable and deep responses in multiple tumor types, paving the way for clinical trials ranging the entire gamut of solid and hematologic tumors.
3
4
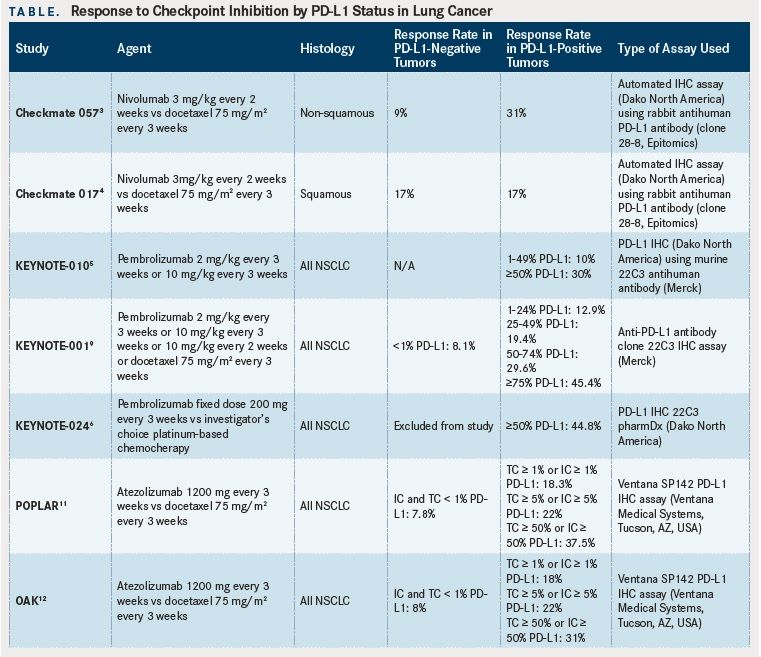
Specific to NSCLC, both nivolumab and pembrolizumab are US Food and Drug Administration (FDA)- approved for use in the second-line treatment of both squamous and non-squamous histologies, after progression on cytotoxic chemotherapy. Nivolumab was approved in non-squamous NSCLC on the basis of the Checkmate 057 study: 582 patients with stage IIIB or IV disease who had received at least 1 prior line of therapy were randomized 1:1 to nivolumab or docetaxel.The study met its primary endpoint of overall survival (OS) improvement (median OS 12.2 months with nivolumab versus 9.4 months with docetaxel; hazard ratio (HR) for death 0.73,P= .002). Checkmate 017 similarly led to the approval of nivolumab for the treatment of squamous NSCLC: 272 patients with stage IIIB or IV disease were randomized in a 1:1 fashion to nivolumab or docetaxel.Median OS improvement was observed in the nivolumab group (9.2 months in the nivolumab group versus 6.0 months in the docetaxel group; HR for death 0.59,P< .001).
5
Pembrolizumab was approved by the FDA for second-line treatment of NSCLC based on the results of the KEYNOTE-010 trial, where 1034 patients with at least 1% PD-L1 tumor cell expression, were randomized in a 1:1:1 design to docetaxel, pembrolizumab 2mg/kg, or pembrolizumab 10mg/kg.Median OS was significantly improved with pembrolizumab (10.4 months in the 2mg/kg arm [HR 0.71,P= .008], 12.7 months in the 10mg/kg arm [HR 0.61,P< .001] versus 8.5 months in the docetaxel arm).
6
More recently, pembrolizumab has been approved by the FDA in the first-line setting based on the findings of the KEYNOTE-024 trial where 305 patients with advanced NSCLC (epidermal growth factor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement-negative) with intratumoral PD-L1 expression of at least 50% were randomized to receive either pembrolizumab or investigator’s choice of platinum-based chemotherapy.The study met its primary endpoint of median progression-free survival (PFS) improvement. The PFS in the pembrolizumab group was 10.3 versus 6.0 months in the chemotherapy cohort (HR for progression or death 0.50,P<.001). Median OS was not reached in either group at the time of publication, but by the second interim analysis, the estimated percentage of patients alive in the pembrolizumab group was 80.2% versus 72.4% in the chemotherapy group (HR for death 0.60,P= .005).
Overall, the introduction of checkpoint inhibitor therapy has changed the landscape of NSCLC treatment in a short time. The challenge, as with any new treatment, is to identify those patients who are likely to respond to this type of therapy. One method used to this end is to examine PD-L1 expression in predicting response to therapy.
PD-L1 as a Biomarker
7
The identification of patients who are likely to benefit from treatment with checkpoint inhibitors has been ongoing since early phase trials. It stands to reason that only those tumors expressing PD-L1 would respond to treatment with these agents. In the earlier, as well as the previously quoted studies, the patients’ tumors were submitted for immunohistochemical analysis of PD-L1 expression on the surface of malignant cells. In a phase I study of nivolumab in solid tumors, intratumoral PD-L1 expression was evaluated on archival or newly obtained paraffin-embedded tissue.Ten NSCLC patients were included in this study. Five out of 10 of these tumors were positive for PD-L1. One patient out of these 5 (20%) had a partial response to nivolumab. None of the patients with negative PD-L1 tumor status had a response to treatment with checkpoint inhibition. All tumors combined, 25 patients had PD-L1 positivity by immunohistochemistry. Of those 9 (36%) had an objective response. The remaining 17 PD-L1-tumor negative patients had no response to treatment with nivolumab. The authors concluded that PD-L1 expression held promise in identifying potential responders to treatment.
4
3
Subsequent larger trials, including the Checkmate 017, Checkmate 057, and KEYNOTE-010 studies also examined PD-L1 intratumoral expression. In Checkmate 017, 225 patients out of 272 with squamous histology NSCLC had quantifiable PD-L1 expression in their tumor specimens.Using predefined expression levels of 1%, 5%, and 10%, the authors observed no statistically significant different response rates between the groups, and concluded that PD-L1 levels were neither predictive of response nor prognostic. Similarly, in the Checkmate 057 study, 455 out of 582 patients with nonsquamous histology NSCLC were found to have quantifiable levels of PD-L1 intratumoral expression.Across all predetermined expression levels (≥1%, ≥5%, and ≥10%), treatment with nivolumab was associated with a statistically significant OS and PFS advantage, though PD-L1 positivity at or above the 5% cut-off had the strongest positive correlation with regard to objective response. Indeed, a trend towards increased response with increasing PD-L1 expression levels was observed.
8
The Checkmate 063 trial was a phase II trial of nivolumab in heavily (2 or more lines of previous therapy) pretreated patients with NSCLC. Out of 117 patients, 76 tumor samples could be assessed for PD-L1 expression.The authors found that both patients with positive or negative PD-L1 tumors achieved an objective response. Partial responses were observed in 52% of patients with PD-L1-positive tumors and 38% of PD-L1-negative tumors. The PD-L1 expression threshold used by the authors was 10%. The authors concluded that while PD-L1 positivity may be useful in predicting response, they identified archival tumor tissue as a potential caveat. PD-L1 expression is known to be dynamic and affected by treatment between the time of biopsy and checkpoint inhibition therapy.
5
9
In the KEYNOTE-010 trial, the findings mirrored those of the Checkmate 017 and 057 trials.Pembrolizumab was strongly associated with improved response at higher PD-L1 expression levels (defined as ≥50%) in non-squamous histologies. It is worth noting, however, that responses were seen in both the PD-L1-positive and negative cohorts. These findings were consistent with those of the phase I KEYNOTE- 001 trial, where response rates in the group of patients with PD-L1 expression levels of 50% or greater were highest at 45.2%.This subgroup of patients had a longer OS and PFS, compared to those patients with less than 50% PD-L1 expression.
10
Analysis of PD-L1 expression in studies of checkpoint inhibition as frontline therapy include the Checkmate 012 trial, which is a recently reported phase I study of nivolumab monotherapy as first-line therapy in advanced NSCLC.Patients with stage IIIB and IV NSCLC were treated with nivolumab 3mg/kg every 2 weeks until disease progression or intolerable toxicity. PD-L1 expression was quantifiable in 88% of tumor specimens. Although it is not a perfect biomarker predictive of response, it appears that stronger PD-L1 expression correlates with increased response rates.
11
12
In the phase II POPLAR study of atezolizumab (Tecentriq, an anti-PD-L1 monoclonal antibody) versus docetaxel in previously treated NSCLC, patient tumor sample PD-L1 expression was scored using immunohistochemistry in both tumor cells (TC) and infiltrating immune cells (IC), using the Ventana SP142 PD-L1 IHC assay (Ventana Medical Systems, Tucson, AZ, USA).This is in contrast to previously mentioned studies where only tumor cell PD-L1 expression was examined. The authors found responses in tumors with TC and/or IC PD-L1 staining. Positive staining was defined as ≥1% PD-L1 expression on TCs or ICs or both. OS was improved with atezolizumab treatment over docetaxel in all groups. A positive correlation between the magnitude of benefit and PD-L1 expression level on TCs and/or ICs was observed. No benefit was observed with atezolizumab over docetaxel in tumors with no PD-L1 expression on either TCs or ICs. The phase III OAK trial has recently reported its primary analysis.TC and IC staining was used in the POPLAR trial. Responses were observed in all patients including those with no PD-L1 expression on TCs and ICs. However, the magnitude of benefit increased with increasing PDL1 expression on either TC or ICs.
13
14
A recent pooled analysis of NSCLC checkpoint inhibitor trials sought to examine the relationship between PD-L1 intratumoral expression and nivolumab response.Nine studies were included in the analysis, for a total of 2012. The authors found that while higher expression of PD-L1 correlates with improved response, patients with tumors harboring less than 1% PD-L1 also derived benefit from treatment. Another pooled analysis found that tumors with 1% or greater PD-L1 expression had a statistically significant increase in objective response rates compared to those with less than 1% (odds ratio [OR] of 2.44; 95% CI; 1.61-3.68) and concluded that an IHC cut-off of 1% could be a useful biomarker, predictive of response to checkpoint inhibition.
15
While all the previous studies examined treatment responses in NSCLC, a recent study looking at efficacy of checkpoint inhibition in small cell lung cancer (SCLC) has been reported. Checkmate 032 is a phase I/II trial examining treatment with nivolumab or the combination of nivolumab and ipilimumab in patients with recurrent SCLC.A total of 216 patients were treated with either nivolumab or nivolumab and ipilimumab at various doses. Overall 10% (10 out of 98) of patients had a response to 3 mg/kg, 23% (14 out of 61) of patients had an objective response to nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, 19% (10 out of 54) of patients had a response to nivolumab 3 mg/ kg plus ipilimumab 1 mg/kg, and 33% (1 out of 3) of patients had a response to the combination of nivolumab 1 mg/kg plus ipilimumab 1 mg/kg. PD-L1 status was assessable in 69% (148 out of 216) of tumor samples. Notably, 39 of these samples were freshly obtained, while the remainder were archival specimens. In a pre-planned exploratory analysis of all subgroups except for the nivolumab 1 mg/ kg plus ipilimumab 1 mg/kg cohort, the authors observed tumor responses regardless of intratumoral PD-L1 expression status. They concluded that PD-L1 status is neither predictive nor prognostic of response in this patient population.
16
The KEYNOTE-028 trial is a phase I trial examining pembrolizumab in patients with extensive-stage SCLC.Eligibility criteria included at least 1% PD-L1 intratumoral expression, which was used as a positivity threshold. Four out of 16 treated patients had a partial response and 1 had stable disease. PD-L1- negative tumors were not included in the study, whose final results are not yet reported.
Overall, though the correlation appears somewhat loose based on the individual trial, the trend that emerges is that patients with PD-L1 expression of 1% or greater may derive benefit from treatment with checkpoint inhibition(Table). This correlation becomes stronger with higher PD-L1 expression levels, making the basis for using a cut-off of 50% or greater expression in the frontline (KEYNOTE-024) trial. Recently, the OAK and POPLAR studies have shifted the PD-L1 expression paradigm to examining expression levels on both tumor cells and infiltrating immune cells. There was a good correlation between PD-L1 expression levels on either cell type and response/OS.
Identifying Patient Responders
Checkpoint inhibition has been the subject of many clinical trials in virtually every type of malignancy. Efficacy has ranged from promising to nil, not only based on tumor type but also demonstrating a broad range within specific diseases. The ongoing challenge is to identify patients who are most likely to respond to treatment and triage patients accordingly. Intratumoral PD-L1 expression was thought to hold some promise in NSCLC, in helping identify those who are likely to respond to checkpoint inhibition. These agents have been found to have activity in both squamous and nonsquamous histologies. With respect to nivolumab, it would appear that PD-L1 expression can be a weak predictor of response: patients with higher levels of expression have a higher likelihood of having a response. However, the absence of PD-L1 expression in nonsquamous histology does not appear to preclude response to treatment. In squamous histology, there does not appear to be a relationship between PD-L1 expression and response to nivolumab. For pembrolizumab, likewise a weak correlation between PD-L1 expression and increasing response rates appears to exist. However, the absence of PD-L1 expression did not preclude response. The current approval of pembrolizumab requires positive testing of PD-L1 using a companion assay while this is not required for nivolumab therapy. For atezolizumab, examination and quantification of PD-L1 expression levels on both tumor cells and infiltrating tumor cells has yielded a positive correlation between response, OS, and PD-L1 expression levels. Patients classified as having negative PD-L1 expression on tumor cells and immune infiltrating cells did no better with atezolizumab than those treated with chemotherapy. The numbers of patients in SCLC trials are too small at this point in time to make any conclusions. Nevertheless, the lack of a crystal clear picture of using PD-L1 as a predictive biomarker in checkpoint inhibition therapy across the board may reflect the heterogeneity of assay testing or the imperfect predictive marker of PD-L1 after all.
17
18
A major pitfall of most studies includes the use of archival tissue, often obtained at the time of initial diagnosis. It is established that PD-L1 levels can vary within tumors and results can be prone to sampling error.Furthermore, PD-L1 expression is dynamic and can fluctuate with treatment with chemotherapy.
19
Another pitfall is the establishment of standardized PD-L1 testing. The most widely used tests are the Ventana SP263, Dako 28-8, and the Dako 22C3 assays.A recent presentation at the American Association for Cancer Research (AACR) showed concordance of PD-L1 expression between these assays. Characterization and standardization of the PD-L1 expression assays is a necessary step not only to assess clinical outcomes but also to allow proper triaging of patients to a treatment that is most likely to benefit them. At this time, it is very clear that further research is needed to determine the extent to which PD-L1 tumor status will play in making treatment decisions. Importantly, standardizing the PD-L1 testing methodology and better consensus of defining PD-L1 positivity will help us understand the role of PD-L1 as a useful predictive biomarker.
References:
- American Cancer Society. Key Statistics for lung cancer. Last updated 05/16/2016. http://www.cancer.org/cancer/lungcancer-non-smallcell/ detailedguide/non-small-cell-lung-cancer-key-statistics. Accessed 11/10/2016.
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity.Curr Opin Immunol.2012;24(2):207-212. doi: 10.1016/j.coi.2011.12.009.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer.N Engl J Med.2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer.N Engl J Med.2015;373(2):123-135. doi: 10.1056/NEJMoa1504627.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial.Lancet.2016;387(10027):1540-1550. doi: 10.1016/ S0140-6736(15)01281-7.
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer.N Engl J Med.2016. [Epub ahead of print]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer.N Engl J Med.2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690.
- Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial.Lancet Oncol.2015;16(3):257-265. doi: 10.1016/S1470-2045(15)70054-9.
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of nonsmall- cell lung cancer.N Engl J Med.2015;372(21):2018-2028. doi: 10.1056/ NEJMoa1501824.
- Gettinger S, Rizvi NA, Chow LQ, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer.J Clin Oncol.2016;34(25):2980- 2987. doi: 10.1200/JCO.2016.66.9929.
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial.Lancet.2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0.
- Barlesi F, Park K, Ciardiello F, et al. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC.Ann Oncol2016;26(suppl 6). doi:10.1093/annonc/mdw1435.1043.
- Aguiar PN, Jr., Santoro IL, Tadokoro H, et al. A pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarker.Immunotherapy.2016;8(9):1011-1019. doi: 10.2217/imt- 2016-0032.
- Passiglia F, Bronte G, Bazan V, et al. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis.Oncotarget.2016;7(15):19738-19747. doi: 10.18632/oncotarget.7582.
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial.Lancet Oncol.2016;17(7):883-895. doi: 10.1016/ S1470-2045(16)30098-5.
- Ott PA, Fernandez MEE, Hiret S, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028.J Clin Oncol.2015; 33 (suppl):7502 (abstr).
- Grigg C, Rizvi NA. PD-L1 biomarker testing for non-small cell lung cancer: truth or fiction?J Immunother Cancer.2016;4:48. doi: 10.1186/s40425-016-0153-x.
- Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer.Sci Rep.2016;6:20090. doi: 10.1038/srep20090.
- Ratcliffe MJ, Sharpe A, Midha A, Barker C, Scorer P and Walker J. A comparative study of PD-L1 diagnostic assays and the classification of patients as PD-L1 positive and PD-L1 negative. In: Proceedings of the 107th Annual Meeting of the American Association for Cancer Research; 2016 Apr 16-20; New Orleans, LA. Abstract LB-094.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More