PD-1 Checkpoint Inhibitors in Pretreated Non-Small Cell Lung Cancer
An overview of the data and rationale that led to the approval of nivolumab and pembrolizumab for NSCLC, with an emphasis on specific patient populations likely to benefit from this approach and future directions for clinical research in this area.
Abstract
The treatment landscape for nonsmall cell lung cancer (NSCLC) has changed dramatically within the past year with the approval of two checkpoint inhibitors, nivolumab and pembrolizumab, for treatment in the second-line setting. Whereas treatment for most patients with NSCLC has traditionally been comprised of chemotherapy, these novel agents raise many intriguing questions about the appropriate timing, combination, and sequencing of immunotherapeutics with chemotherapy. Selecting patients likely to benefit from anti–PD-1 therapy and determining when they should receive these agents will be an important research focus in the immediate future. This article briefly reviews the background data and rationale that led to the approval of nivolumab and pembrolizumab for NSCLC, with an emphasis on specific patient populations likely to benefit from this approach and future directions for clinical research in this area.
Background
Lung cancer continues to be the leading cause of cancer-related deaths in the United States and a significant national health concern.1 The majority of patients with newly diagnosed nonsmall lung cancer (NSCLC) have metastatic disease and are not eligible for curative surgical or combined modality treatments. For decades, the mainstay of treatment at the time of initial diagnosis has been a platinum doublet-based combination chemotherapy regimen. Response rates with first-line chemotherapy have typically been approximately 30%, with median overall survival (OS) rates of approximately 10 to 12 months.2,3Response rates to second-line chemotherapy agents such as docetaxel have been approximately 5% to 10% with median OS of about 7 months.4,5A smaller subset of patients with NSCLC have tumors with driver mutations, such as a mutation in theEpidermal Growth Factor Receptor (EGFR)or an Echinoderm microtubule like protein 4ALK translocation; these patients are treated with tyrosine kinase inhibitors aimed at these specific alterations. These targeted drugs improved response rates and possibly survival times compared to standard chemotherapy, but typically resistance emerges with continued treatment.6,7Given the plateau in efficacy with chemotherapy and the expectation of resistance development with targeted agents, there has been continued interest in developing novel therapeutics for NSCLC, including immunotherapy agents.
The capacity of the host immune system to control and sometimes eradicate tumors has been known for decades. The use of cytokines, most notably IL-2 in melanoma and renal cell carcinoma (RCC), had shown the possibility of achieving tumor control, however, this was not applicable to most tumor types and the approach suffered from poor tolerability. 8 Lung cancer, in particular, was felt to be a nonimmunogenic site, a term coined to indicate futility of immunological approaches for treatment of this disease based on prior studies including vaccine trials.9,10This perspective has changed entirely with the introduction of checkpoint inhibitors, and in particular those targeting the Programmed Cell Death 1 (PD-1) pathway. PD-1 is a type I transmembrane surface protein expressed on the surface of activated T cells. When the PD-1 receptor binds to one of its ligands (PD-L1 or PD-L2) it leads to down-regulation of T-cell activity through intracellular inhibitory signaling, thus keeping T-cell activation in check.11Tumor cells and inflammatory cells in the tumor microenvironment can express PD-L1 and PD-L2, and interaction with PD-1 on T cells can enable tumor growth and persistence through immune evasion.12Nivolumab and pembrolizumab are both monoclonal antibodies (mAb) capable of blocking the binding of PD-1 to its ligands, thus maintaining an activated T-cell status within the tumor microenvironment. Both of these antibodies have been shown to have anti-tumor activity across a wide range of malignancies, including NSCLC.
Clinical Activity of AntiPD-1 Agents in NSCLC
Nivolumab was first shown to have anti-tumor activity in a phase I study involving 39 patients with solid tumors, initially reported in 2010. This cohort included 6 patients with NSCLC, one of whom experienced tumor regression.13Given the promising results of this pilot study, an expanded phase I trial was initiated, enrolling 296 patients, including 122 with relapsed/refractory advanced NSCLC (other arms included melanoma, RCC, colorectal cancer, prostate cancer).14Objective responses were noted in 33% of patients with squamous cell NSCLC (Sq-NSCLC) and 12% of patients with non-squamous NSCLC (nonSq-NSCLC), leading to considerable excitement given the historical response rates of chemotherapy in this patient population. Subsequently 2 large, randomized phase III trials, 1 enrolling patients with squamous cell histology (CheckMate-017) and 1 enrolling patients with non-squamous cell histology (CheckMate-057), were initiated. Nivolumab dose of 3 mg/kg every 2 weeks was chosen for these trials.
Checkmate-017 randomized 272 patients with advanced Sq-NSCLC whose disease had progressed after first-line platinum-doublet chemotherapy to receive either nivolumab or docetaxel, with a primary endpoint of OS. The results were published in May 2015. Patients in the nivolumab arm had an improvement in OS (median OS 9.2 months vs 6.0 months) and many of the patients on nivolumab achieved durable responses.15Of note, 83% of the patients had evaluable tumor samples for PD-L1 staining. PD-L1 positivity (defined in 3 groups, ≥1%, ≥5%, ≥10%) was not prognostic or predictive for the efficacy outcomes as defined by the protocol. Based on the results of this trial, the FDA approved nivolumab for patients with Sq-NSCLC who had progressed after first-line chemotherapy in March 2015.
CheckMate-057 had a similar design as the 017 study except that it enrolled patients with nonSq-NSCLC. In this trial, 582 patients were randomized to receive either nivolumab or docetaxel, with a primary endpoint of OS. Similarly to CheckMate- 017, this study found that nivolumab was superior to docetaxel, with an improvement in median OS (12.2 months vs 9.4 months) and a subset of patients obtaining durable responses to nivolumab.16Seventy-eight percent of patients had tumors evaluable for PD-L1 staining, and in this study PD-L1 positivity was predictive of response to nivolumab therapy. However, since clinical efficacy compared to docetaxel was demonstrated in an “all-comers” population regardless of PD-L1 status, the FDA approved nivolumab for all patients with nonSq-NSCLC who had progressed after first-line chemotherapy in October 2015. The first report of the anti-tumor activity of pembrolizumab came at the ASCO Annual Meeting in 2012. This initial trial had 17 evaluable patients, including 5 patients with NSCLC (other tumor types were melanoma, colorectal, and carcinoid/neuroendocrine tumor).17The acceptable safety profile and signal of clinical efficacy in this study led to large, expanded phase I trials in melanoma and NSCLC. The KEYNOTE-001 trial was an international phase I study which enrolled 495 patients with advanced NSCLC. The trial was designed to develop and validate a PD-L1 expression level which would predict benefit from pembrolizumab therapy, in addition to evaluating safety and clinical efficacy endpoints. In contrast to the CheckMate trials noted above, all patients were required to have a biopsy evaluable for PD-L1 prior to enrollment, and patients with no prior therapy were allowed to enroll. The results were published in May 2015, and revealed tolerable safety and significant clinical efficacy for pembrolizumab, especially in patients with high expression of PD-L1.18The investigators defined 3 subgroups based on PD-L1 expression proportion score (PS): PS ≥50% (23% of patients), PS 1% to 49% (38% of patients), and PS <1% (39% of patients). For all patients, the overall response rate was 19.4% (18% in previously treated patients and 24.8% in untreated patients) and median OS was 12.0 months, similar to the results from the CheckMate studies discussed above. However, within the group of patients with a PS ≥50% that included treatment-naïve and previously treated subjects, the response rate was 45.2% and median OS was not reached, demonstrating a remarkable duration of response. Based upon these results, the FDA approved pembrolizumab for patients with advanced NSCLC which expresses PD-L1 (PS ≥50%) who have progressed beyond first-line therapy.
KEYNOTE-010 is a randomized phase III trial that compared pembrolizumab to docetaxel in previously treated patients with advanced NSCLC. Two different dose levels of pembrolizumab were evaluated. Overall, 1034 patients were randomized to 3 different arms, docetaxel, pembrolizumab at 2 mg/kg and 10 mg/kg every 3 weeks. The co-primary endpoints were OS and progression-free survival (PFS) in the total population and in patients with PD-L1 expression on at least 50% of tumor cells. As reported, the median OS was superior in both pembrolizumab arms compared with docetaxel (median OS, 10.4 months with pembrolizumab at 2 mg/kg, 12.7 months for pembrolizumab at 10 mg/kg, and 8.5 months with docetaxel).19In the subset of patients with tumor PD-L1 expression of >50%, both the OS and PFS were superior to docetaxel (HR for OS, 0.54 with 2 mg/kg and 0.50 with 10 mg/kg and HR for PFS 0.59 for both dose levels).
These studies have established the efficacy of checkpoint inhibitors in patients with previously treated NSCLC. Both nivolumab and pembrolizumab have been investigated in treatment-naïve patients in a randomized comparison against frontline platinum-doublet chemotherapy. The results of these trials have not been officially reported but are highly anticipated.
Toxicity
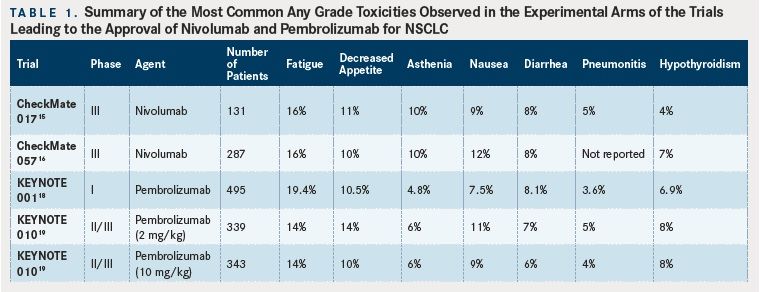
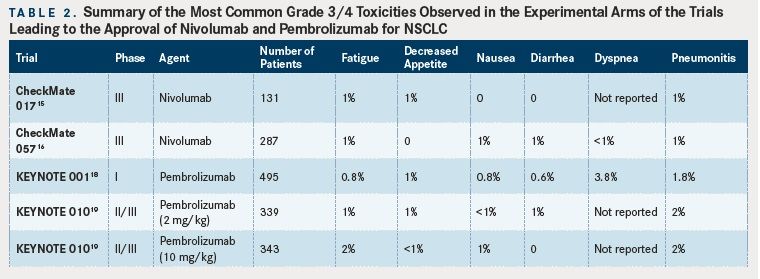
(Tables 1 and 2).
Safety data from the studies described above are generally encouraging for these antiPD-1 mAbs, as they are typically better tolerated than single-agent cytotoxic chemotherapy with rates of grades 3 to 4 adverse events of less than 10% (compared with approximately 50% with chemotherapy). However, clinicians must be wary of autoimmune adverse events such as pneumonitis or colitis which can have a sudden onset and be life-threatening. Rapid recognition of these events and administration of high dose corticosteroids are required to reduce morbidity and mortality. Given the potential for inducing immune-related adverse events based on the mechanism of action for these drugs, patients with active autoimmune diseases were not eligible for any of the clinical trials described above due to fear of exacerbation of their underlying disease. However, recently published data on the use of a checkpoint blockade agent (ipilimumab, which targets cytotoxic leukocyte antigen-4 [CTLA4]) in melanoma patients with concurrent autoimmune disease (eg, rheumatoid arthritis, ulcerative colitis) suggests that a minority (27% in this case series) of patients will experience a flare of their autoimmune disease and that autoimmune toxicities can be safely managed with clinical vigilance.20Thus, the risks and benefits of using antiPD-1 agents in patients with NSCLC must be discussed and considered on a case-by-case basis. However, we feel that for most patients the possible benefit of durable response to treatment, although unlikely, outweighs the risk of autoimmune toxicity, which is also unlikely. The tables provide a summary of reported side effects from the studies discussed above
In our experience, the timing of autoimmune toxicity incidence is unpredictable, in that some patients experience toxicities early in the course of treatment and some much later. We also engage experts from other disciplines such as gastroenterology, pulmonary, and endocrinology early in the comanagement of autoimmune adverse events. Lastly, and perhaps most importantly, communication with the patient and education regarding the importance of notifying the care team about any new symptoms is crucial to identify and manage autoimmune toxicity in a timely manner.
Selecting NSCLC Patients for AntiPD-1 Therapy
For most patients with NSCLC who are eligible for second-line therapy, treatment with nivolumab or pembrolizumab is a reasonable option. Nivolumab can be given without any PD-L1 testing, making it an attractive option for use in patients with scant or unavailable tumor tissue who are ready to begin treatment. Within the Sq-NSCLC population studied in CheckMate-017, no significant differences in efficacy signal were seen in patient subsets based on their PD-L1 expression. In non-Sq NSCLC, however, the OS hazard ratios for certain subgroups did not favor nivolumab; these subgroups included never smokers, patients with EGFR-activating mutations, and patients receiving third-line therapy on the trial. One rationale for poor nivolumab response in never smokers and patients with activating EGFR mutations is the expectation that tumors with higher mutational burden (often found in smokers due to the carcinogenic effects of tobacco smoke) will develop more neoantigens, thus increasing their immunogenicity, and making them more susceptible to immunotherapy.21
The discussion regarding PD-L1 as a biomarker for patient selection is evolving. There are many documented problems with using PD-L1 as a biomarker to predict response, including intratumoral PD-L1 heterogeneity, the dynamic nature of PD-L1 expression, and the variability between available assays.22Efforts such as the Blueprint Working Group, which is a collaborative effort between professional organizations including AACR and ASCO, the FDA, and various industry partners, are underway to help clarify the strengths/weaknesses of PD-L1 testing as a biomarker for clinicians and patients. Part of the difficulty with this discussion is that every major drug company with an antiPD-1 or PD-L1 antibody has chosen to perform their test with a different antibody, different platform, and varying cut-offs for defining positive expression. What has become clear regardless of the antibody assay or specific drug used is the fact that high expression of PD-L1 is associated with better tumor response and better patient survival. However, a modest proportion of patients with low or negative expression of this marker (ie, PD-L1 negative) seem to respond as well. Thus, PD-L1 expression alone is likely not the best biomarker for patient selection. For patients with strongly positive PD-L1 testing with the DAKO 22C3 IHC assay (eg, ≥50%), the currently available data suggest a high expectation for tumor response to treatment with pembrolizumab. At this time, expert opinion supports the use of an FDA-approved PD-L1 assay to help guide management, however, this testing is most useful only in cases of very high PD-L1 expression (ie, very high expression suggests increased likelihood of response, but low or negative expression does not rule out possible response). This continues to be an area of active research and debate.
Future Directions for PD-1 Inhibitors in NSCLC
Given the relative success of nivolumab and pembrolizumab in relapsed/refractory clinical settings, and based on the first-line cohort data from KEYNOTE-001, multiple clinical trials featuring antiPD-1 mABs as first-line monotherapy for NSCLC have been initiated, as well as multiple trials featuring combination immunotherapy drug approaches in all lines of therapy. On August 5, 2016, Bristol-Myers Squibb announced that the CheckMate-026 trial, evaluating nivolumab monotherapy versus platinum-doublet chemotherapy for patients with PD-L1 positive tumors (≥5%), did not meet its primary endpoint of improved PFS for the nivolumab arm.23Another first-line trial, CheckMate-227, is currently accruing: this trial is investigating nivolumab versus nivolumab plus ipilimumab versus platinum-doublet chemotherapy in PD-L1positive patients, and nivolumab plus ipilimumab versus nivolumab plus platinumdoublet chemotherapy versus platinum-doublet chemotherapy in PD-L1 negative patients, with OS and PFS as co-primary endpoints.
Merck, on the other hand, delivered an exciting press release on June 16, 2016, announcing that the KEYNOTE-024 trial investigating pembrolizumab versus platinum-doublet chemotherapy in the first line for patients with PD-L1positive tumors (≥50%) met its primary endpoint of improvement in PFS, as well as a secondary endpoint of improvement in OS.24These results have not been publicly presented at the time of this writing. It seems likely that PD-1 monotherapy will soon be entering clinical practice as first-line therapy for a subset of patients with NSCLC, and ongoing research results are eagerly anticipated.
We also anticipate the emergence of combination treatment protocols with 2 or perhaps even 3 different immune modulating agents. Early results from some of these studies have been published or reported. As our understanding of the tumor immune system interaction increases, we can design trials with several different drugs with the expectation that each drug would alter or modulate different aspects of the immune system and tumor microenvironment, thus hypothetically leading to better tumor control. However, it is not clear how the addition of multiple immunotherapy agents into combination regimens might affect the toxicity profile; the hope is that these regimens will be well-tolerated based on the experience with anti–PD-1 agents thus far. Results of phase III trials utilizing the potential of cytotoxic chemotherapy when combined with a checkpoint inhibitor are also anticipated.
Conclusions
This is an exciting time in NSCLC clinical practice and research. In 2015, 2 new immunotherapy agents, nivolumab and pembrolizumab, were approved by the FDA for the treatment of NSCLC by demonstrating a meaningful survival advantage versus chemotherapy, marking a true advance in the realm of NSCLC therapeutics. The field of biomarker investigation and selection is a highly active area of research, as much is unknown regarding why, despite impressive responses in certain patient populations, the majority of patients with NSCLC do not respond to PD-1 blockade. Numerous trials involving immunotherapy agents in every line of treatment of NSCLC are ongoing and feature checkpoint inhibitors beyond nivolumab and pembrolizumab, including antiPD-L1 antibodies such as atezolizumab, durvalumab, and avelumab and anti-CTLA4 antibodies such as ipilimumab and tremelimumab. Many combination trials featuring 2 immunotherapy agents aimed at different checkpoints, or a checkpoint inhibitor plus an agent aimed at augmenting immune response through another mechanism (eg, epigenetic therapy, metabolic targets such as IDO1) are also ongoing.
This is an evolving field with many new exciting and promising agents entering clinical practice. Appropriate patient selection to maximize benefit and minimize potential toxicities remains a challenge and one that requires more research. The hope is that all of these efforts will lead to better treatments for our patients and a better understanding of the interaction between the tumor and the immune system.
References:
- Siegel RL, Miller KD, Jemal A.: Cancer statistics, 2016.CA Cancer J Clin.2016;66(1):7-30. doi: 10.3322/caac.21332.
- Schiller JH, Harrington D, Belani CP, et al; Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer.N Engl J Med. 2002;346(2):92-98.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer.J Clin Oncol.2000;18(12):2354-2362.
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 non-small cell lung cancer study group.J Clin Oncol.2000;18(12):2354- 2362.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22(9):1589-1597.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers.Clin Cancer Res.2013;19(8):2240-2247. doi: 10.1158/1078-0432.CCR- 12-2246.
- Solomon BJ, Mok T, Kim DW, et al; PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer.N Engl J Med.2014;371(23):2167-2177. doi: 10.1056/NEJMoa1408440.
- Atkins MB. Interleukin-2: clinical applications.Semin Oncol.2002;29(3)(suppl 7):12-17.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age.Nature.2011;480(7378):480-489. doi: 10.1038/nature10673.
- Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3- positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial.Lancet Oncol.2016;17(6):822-835. doi: 10.1016/S1470-2045(16)00099-1.
- Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy.J Leukoc Biol.2013;94(1):25-39. doi: 10.1189/jlb.1212621.
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment.Nat Rev Immunol.2008;8(6):467-477. doi: 10.1038/nri2326.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent antiprogrammed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates.J Clin Oncol.2010;28(19):3167-3175. doi: 10.1200/JCO.2009.26.7609.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of antiPD-1 antibody in cancer.N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer.N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer.N Engl J Med.2015;373(17):1627-39. doi: 10.1056/NEJMoa1507643.
- Patnaik A, Kang SP, Rasco D, et al. Phase I study of pembrolizumab (MK-3475; antiPD-1 monoclonal antibody) in patients with advanced solid tumors.Clin Cancer Res.2015;21(19):4286-4293. doi: 10.1158/1078-0432.CCR-14-2607.
- Garon EB, Rizvi NA, Hui R, et al; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer.N Engl J Med. 2015;372(21):2018- 2028. doi: 10.1056/NEJMoa1501824.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial.Lancet. 2016;387(10027):1540-1550. doi: 10.1016/ S0140-6736(15)01281-7.
- Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders.JAMA Oncol. 2016;2(2):234-240. doi: 10.1001/jamaoncol.2015.4368.
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer.Science. 2015;348(6230):124-128. doi: 10.1126/science.aaa1348.
- McLaughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer.JAMA Oncol.2016;2:46-54. doi: 10.1001/jamaoncol.2015.3638.
- Bristol-Myers Squibb. Bristol-Myers Squibb announces top-line results from CheckMate-026, a phase 3 study of Opdivo (nivolumab) in treatment naive patients with advanced non-small cell lung cancer. Press release, August 5, 2016.http://investor.bms.com/investors/news-and-events/press-releases/pressrelease- details/2016/Bristol-Myers-Squibb-Announces-Top-Line-Results-from- CheckMate--026-a-Phase-3-Study-of-Opdivo-nivolumab-in-Treatment-Nave- Patients-with-Advanced-Non-Small-Cell-Lung-Cancer/default.aspx
- Merck: Merck’s KEYTRUDA (pembrolizumab) demonstrates superior progressionfree and overall survival compared to chemotherapy as first-line treatment in patients with advanced non-small cell lung cancer, 2016.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
